53-03-2
| 中文名 | 泼尼松 |
|---|---|
| 英文名 | prednisone |
| 中文别名 |
强的松
强的松,去氢可的松 |
| 英文别名 |
MFCD00003608
In-Sone MOPP deltae EINECS 200-160-3 17,21-Dihydroxypregna-1,4-diene-3,11,20-trione Deltra δ1-Cortisone Delcortin Cotone D1-Dehydrocortisone deltacortisone Cortan (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(hydroxyacetyl)-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthrene-3,11(6H)-dione 1,4-Pregnadiene-17a,21-diol-3,11,20-trione Wojtab Precortal Deltisone u6020 deltacortene Prednisone (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(hydroxyacétyl)-10,13-diméthyl-7,8,9,10,12,13,14,15,16,17-décahydro-3H-cyclopenta[a]phénanthrène-3,11(6H)-dione D1-Cortisone (8S,9S,10R,13S,14S,17R)-17-Hydroxy-17-(hydroxyacetyl)-10,13-dimethyl-7,8,9,10,12,13,14,15,16,17-decahydro-3H-cyclopenta[a]phenanthren-3,11(6H)-dion δ1-Dehydrocortisone |
| 描述 | Prednisone (Adasone)是皮质类固醇试剂,有免疫抑制作用。 |
|---|---|
| 相关类别 | |
| 参考文献 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 573.7±50.0 °C at 760 mmHg |
| 熔点 | 236-238 °C(lit.) |
| 分子式 | C21H26O5 |
| 分子量 | 358.428 |
| 闪点 | 314.8±26.6 °C |
| 精确质量 | 358.178009 |
| PSA | 91.67000 |
| LogP | 1.57 |
| 外观性状 | 白色结晶粉末 |
| 蒸汽压 | 0.0±3.6 mmHg at 25°C |
| 折射率 | 1.604 |
| 储存条件 | 主要用于各种急性严重细菌感染,严重过敏性疾病,胶原性疾病(红斑狼疮、结节性动脉周围炎等),风湿病、类风湿性关节炎、肾病综合征,严重支气管哮喘、血小板减少性紫癜、粒细胞减少症、急性淋巴性白血病、各种肾上腺皮质功能不全症、剥脱性皮炎、天皰疮、神经性皮炎、湿疹等。 |
| 分子结构 | 1、 摩尔折射率:94.08 2、 摩尔体积(m3/mol):273.6 3、 等张比容(90.2K):757.0 4、 表面张力(dyne/cm):58.5 5、 极化率(10-24cm3):37.29 |
| 计算化学 | 1、 疏水参数计算参考值(XlogP):1.5 2、 氢键供体数量:2 3、 氢键受体数量:5 4、 可旋转化学键数量:2 5、 互变异构体数量:27 6、 拓扑分子极性表面积(TPSA):91.7 7、 重原子数量:26 8、 表面电荷:0 9、 复杂度:764 10、同位素原子数量:0 11、确定原子立构中心数量:6 12、不确定原子立构中心数量:0 13、确定化学键立构中心数量:0 14、不确定化学键立构中心数量:0 15、共价键单元数量:1 |
| 更多 | 1. 性状:白色或类白色结晶性粉末。无气味。略有持续性苦味 2. 密度(g/mL,25/4℃): 未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):233~235 5. 沸点(ºC,常压):未确定 6. 沸点(ºC, 13.33kpa): 7. 折射率: 未确定 8. 闪点(ºC): 未确定 9. 比旋光度(º, C=1, DIOXANE):172 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC): 未确定 12. 饱和蒸气压(kPa,60ºC): 未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:1g产品溶于约150ml乙醇、约200ml氯仿,微溶于甲醇、二氧六环,极微溶于水。 |
|
Section 1. Chemical Product and Company Identification Prednisone Common Name/ Trade Name Prednisone Section 4. First Aid Measures Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at least
Eye Contact 15 minutes. Cold water may be used. WARM water MUST be used. Get medical attention if irritation occurs. Wash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Skin Contact Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Not available. Serious Inhalation Do NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an Ingestion unconscious person. If large quantities of this material are swallowed, call a physician immediately. Loosen tight clothing such as a collar, tie, belt or waistband. Not available. Serious Ingestion Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Not available. Flash Points Not available. Flammable Limits These products are carbon oxides (CO, CO2). Products of Combustion Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Risks of explosion of the product in presence of mechanical impact: Not available. Explosion Hazards in Slightly explosive in presence of open flames and sparks. Presence of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media LARGE FIRE: Use water spray, fog or foam. Do not use water jet. and Instructions Material in powder form, capable of creating a dust explosion. As with most organic solids, fire is possible at elevated Special Remarks on temperatures Fire Hazards Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust Special Remarks on explosion hazard. Explosion Hazards Section 6. Accidental Release Measures Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading Small Spill water on the contaminated surface and dispose of according to local and regional authority requirements. Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on Large Spill the contaminated surface and allow to evacuate through the sanitary system. Prednisone Section 7. Handling and Storage Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not Precautions breathe dust. Wear suitable protective clothing. If you feel unwell, seek medical attention and show the label when possible. Keep away from incompatibles such as oxidizing agents. Keep container tightly closed. Keep container in a cool, well-ventilated area. Material should be stored at a Storage temperature less than 40 deg C., preferably between 15-30 deg. C. Section 8. Exposure Controls/Personal Protection Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below Engineering Controls recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE of a Large Spill handling this product. Not available. Exposure Limits Section 9. Physical and Chemical Properties Solid. (Solid crystalline powder.)Odorless. Physical state andO dor appearance Bitter. Aftertaste. Taste 358.43 g/mole Molecular Weight White or nearly white. Color Not available. pH (1% soln/water) Not available. Boiling Point Decomposition temperature: 230°C (446°F) - 235 C. Melting Point Not available. Critical Temperature Not available. Specific Gravity Not applicable. Vapor Pressure Not available. Vapor Density Not available. Volatility Not available. Odor Threshold Not available. Water/Oil Dist. Coeff. Not available. Ionicity (in Water) Not available. Dispersion Properties Very slightly soluble in cold water. Solubility Slightly soluble in methanol. 1 g soluble in 150 ml alcohol. 1 g soluble in 200 ml chloroform. Section 10. Stability and Reactivity Data The product is stable. Stability Not available. Instability Temperature Conditions of Instability Excess heat, incompatible materials. Incompatibility with various Reactive with oxidizing agents. substances Prednisone Non-corrosive in presence of glass. Corrosivity Not available. Special Remarks on Reactivity Not available. Special Remarks on Corrosivity Will not occur. Polymerization Section 11. Toxicological Information Inhalation. Ingestion. Routes of Entry Toxicity to AnimalsLD50: Not available. LC50: Not available. Chronic Effects on Humans CARCINOGENIC EFFECTS: 3 (Not classifiable for human.) by IARC. MUTAGENIC EFFECTS: Mutagenic for bacteria and/or yeast. Slightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Other Toxic Effects on Humans Not available. Special Remarks on Toxicity to Animals May cause adverse reproductive effects and cancer based on animal test data. Special Remarks on Chronic Effects on Humans May affect genetic material (mutagenic). Human: small amount excreted in maternal milk. Acute Potential Health Effects: Special Remarks on other Skin: May cause skin irritation. May cause skin sensitization, an allergic reaction. Toxic Effects on Humans Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritation. Ingestion: May cause gastritis with nausea, vomiting, abdominal distention, ulcerative esophagitis, increased sweating. May affect metabolism and cause disturbance of electrolyte balance which is manifest in the retention of sodium and water, with edema and hypertension, and in the increased excretion of potassium with the possibility of hypokalaemic alkalosis. Cardiac faillure may be induced in sensitive individuals. Chronic Potential Health Effects: Skin: Prolonged or repeated skin contact may cause sensitization, an allergic reaction. Eyes: Prolonged contact with eyes may affect vision (cataracts) Inhalation/Ingestion: May cause anaphylaxis, an allergic reaction. Prolonged or repeated inhalation or ingestion of corticosteroids may also cause thinning of the skin, inpaired wound healing and other dermatologic reactions (erythema, petechiae and ecchymoses). Ingestion: Prolonged or repeated ingestion of corticosteroids may cause nausea, vomiting, abdominal distention, peptic ulcer with possible perforation and hemorrhage, possible hypersensitization, swelling of the feet and lower legs due to electrolyte imbalance and sodium retention, irregular hearbeat, acute adrenal insufficiency, osteoporosis, muscle weakness, loss of muscle mass, vertebral compression fractures, pathologic fracture of long bones, aseptic necrosis of femoral and humeral heads, effect glucose tolerance (causing decreased carbohydrate tolerance resulting in manifestations of latent Diabetes melliltus, increased requirements for insulin or oral hypoglycemifcagents in diabetes), the liver, pancreas (pancreatitis), eyes/vision (cataracts, glaucoma, increased intraocular pressure), behavior/central nervous system/nervous system (mental disturbances such as psychosis, euphoria, depression, vertigo, headache, convulsions, increased intracranial pressure with papilledema). It may also effect the immune system (immunosuppressant). Repeated large doses of corticosteroids may produce Cushingoid symptoms typical of hyperactivity of the adrenal cortex, with moon-face, sometimes hirsutism, buffalo hump, flushing, increased bruising, ecchymoses, striae, and acne. Medical Conditions Aggravated by Exposure: Hypersensivity to material, ocular herpes simplex, active alcoholism, AIDS or HIV infection, heart disease or hypertension, diabetes mellitus, myasthenia gravis, impaired kidney or liver function, esophagitis, gastritis or peptic ulcer, chickenpox or measles (including recent exposure), recent intestinal surgery, tuberculosis, and system fungal infections. Note: The above information came from Physicians GenRx - Drug Information, 1995 or was extrapolated from information for Corticosteriods found in a reference called Martindale. Prednisone Section 12. Ecological Information Not available. Ecotoxicity Not available. BOD5 and COD Products of Biodegradation Possibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The product itself and its products of degradation are not toxic. Toxicity of the Products of Biodegradation Not available. Special Remarks on the Products of Biodegradation Section 13. Disposal Considerations Waste must be disposed of in accordance with federal, state and local environmental control Waste Disposal regulations. Section 14. Transport Information Not a DOT controlled material (United States). DO T Cl assi fi cati on Not applicable. Identification Not applicable. Special Provisions for Transport DO T (Pi ctograms) Section 15. Other Regulatory Information and Pictograms New Jersey: Prednisone Federal and State TSCA 8(b) inventory: Prednisone Regulations California prop. 65: This product contains the following ingredients for which the State of California has found to California cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS No. Other Regulations 200-160-3). Canada: Listed on Canadian Non-Domestic Substance List (NDSL). China: Not listed on National Inventory. Japan: Listed on National Inventory (ENCS). Korea: Listed on National Inventory (KECI). Philippines: Not listed on National Inventory (PICCS). Australia: Listed on AICS. WHMIS (Canada) Not controlled under WHMIS (Canada). Other Classifications Not availableNot applicable. DSCL (EEC) Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity0 Specific hazard Personal Protection E Prednisone WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG(Canada) (Pictograms) ADR (Europe) (Pictograms) Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Prednisone CALL (310) 516-8000 Notice to Reader All chemicals may pose unknown hazards and should be used withcaution. This Material Safety Data Sheet (MSDS) applies only to the material as packaged. If this product is combined with other materials, deteriorates, or becomes contaminated, it may pose hazards not mentioned inthis MSDS. It shall be the user's responsibility to develop proper methods of handling and personal protection based onthe actual SECTION 16 - ADDITIONAL INFORMATION N/A |
|
毒理学数据: 1 、急性毒性:女人经口TDLo:2400ug/kg/2D-I;男人经口TDLo:857ug/kg;男人经口LDLo:400ug/kg;女人TDLo:113mg/kg; 小鼠腹腔LD50:135 mg/kg;小鼠皮下LD50:101 mg/kg;小鼠肌内LD50:600 mg/kg 2、致癌性:大鼠腹腔TDLo:860mg/kg/26W-I 3、繁殖毒性:雌性小鼠皮下TDLo:24mg/kg,13-18天后受孕 4 、致突变microorganismsTEST系统:细菌-鼠伤寒沙门氏菌:3333ug/plate; 突变microorganismsTEST系统:细菌-鼠伤寒沙门氏菌:333ug/plate;CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| 符号 |

GHS08 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H361 |
| 警示性声明 | P281 |
| 个人防护装备 | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| 危害码 (欧洲) | Xn:Harmful |
| 风险声明 (欧洲) | R63 |
| 安全声明 (欧洲) | S36/37/39-S45 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| RTECS号 | TU4154100 |
| 海关编码 | 2937210000 |
|
~88% 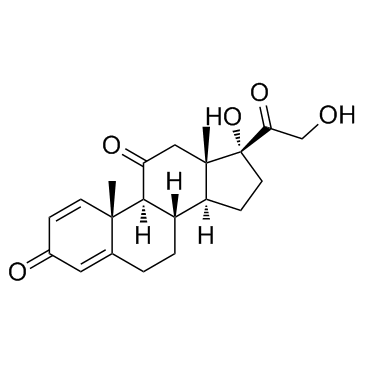
53-03-2 |
| 文献:Wang, Shao-Min; Zhang, Yan-Bing; Liu, Hong-Min; Yu, Guo-Bin; Wang, Ke-Rang Steroids, 2007 , vol. 72, # 1 p. 26 - 30 |
|
~% 
53-03-2 |
| 文献:Kruszewska; Chmielowiec; Uszycka-Horawa Farmaco, 1996 , vol. 51, # 12 p. 809 - 810 |
|
~% 
53-03-2 |
| 文献:Hogg et al. Journal of the American Chemical Society, 1955 , vol. 77, p. 4436 |
|
~% 
53-03-2 |
| 文献:Hogg et al. Journal of the American Chemical Society, 1955 , vol. 77, p. 4436 |
|
~% 
53-03-2 |
| 文献:Schering Corp. Patent: US2837464 , 1955 ; Full Text Show Details Herzog et al. Tetrahedron, 1962 , vol. 18, p. 581,588 |
|
~% 
53-03-2 |
| 文献:Szpilfogel et al. Recueil des Travaux Chimiques des Pays-Bas, 1956 , vol. 75, p. 475,480 |
|
~% 
53-03-2 |
| 文献:Kane; Tsuji Journal of Pharmaceutical Sciences, 1983 , vol. 72, # 1 p. 30 - 35 |
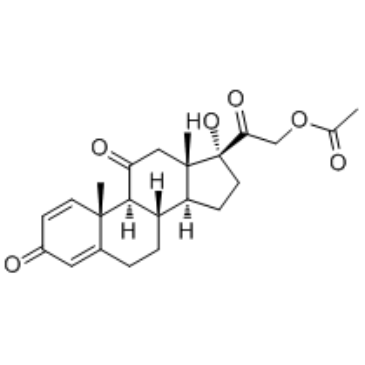
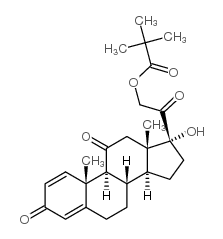
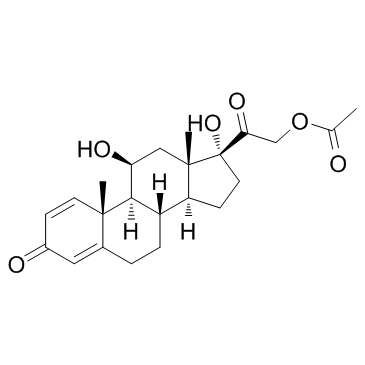
| 上游产品 7 | |
|---|---|
| 下游产品 2 | |
| 海关编码 | 2937210000 |
|---|


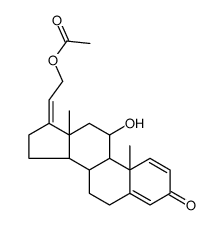
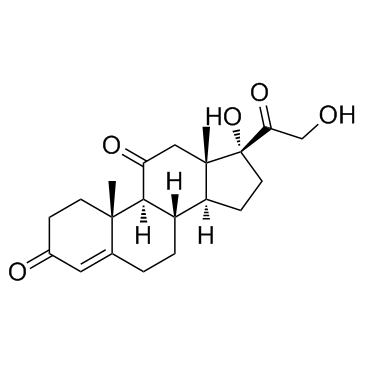
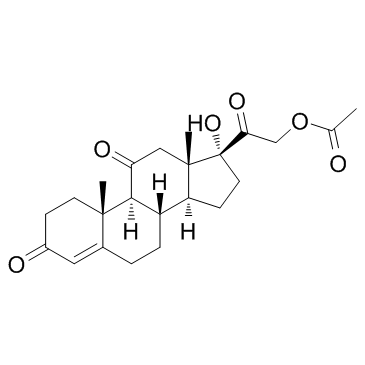
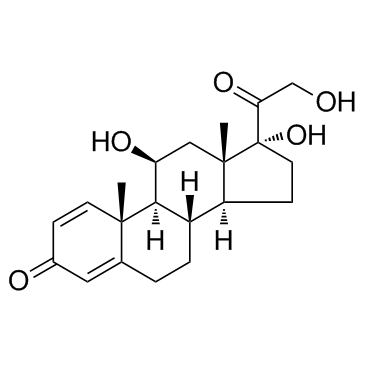
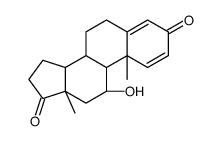
![(9S,10R,13S,14S,17R)-17-hydroxy-10,13-dimethyl-3,11-dioxo-6,7,8,9 ,12,14,15,16-octahydrocyclopenta[a]phenanthrene-17-carboxylic aci d结构式](https://image.chemsrc.com/caspic/404/78261-67-3.png)