(S)-(+)-吡咯烷-3-甲酸
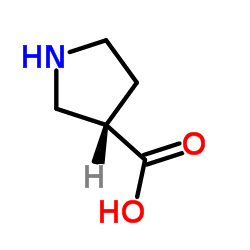
(S)-(+)-吡咯烷-3-甲酸结构式

|
常用名 | (S)-(+)-吡咯烷-3-甲酸 | 英文名 | (S)-pyrrolidine-3-carboxylic acid |
|---|---|---|---|---|
| CAS号 | 72580-53-1 | 分子量 | 115.131 | |
| 密度 | 1.186 | 沸点 | 252 ºC | |
| 分子式 | C5H9NO2 | 熔点 | 185-191°C | |
| MSDS | 美版 | 闪点 | 106 ºC |
|
Diastereocontrolled synthesis of enantioenriched 3,4-disubstituted beta-prolines.
J. Org. Chem. 72 , 398, (2007) Enantioenriched 3,4-disubstituted beta-prolines have been prepared with a high diastereocontrol through a carbometalation reaction or through a domino Michael addition/carbometalation reaction. |
|
|
Beta-proline analogues as agonists at the strychnine-sensitive glycine receptor.
J. Med. Chem. 35 , 233, (1992) 3-Carboxy-3,4-dehydropyrrolidine was found to bind with affinity equal to that of glycine in a [3H]strychnine binding assay. Simple substitution of the 1-, 2-, 4-, or 5-position resulted in marked loss of affinity. A decline in affinity was also found upon en... |
|
|
Endomorphin-1 analogues containing beta-proline are mu-opioid receptor agonists and display enhanced enzymatic hydrolysis resistance.
J. Med. Chem. 45(12) , 2571-8, (2002) In this paper we describe the synthesis and affinity toward the mu-opioid receptor of some tetrapeptides obtained from endomorphin-1, H-Tyr-Pro-Trp-Phe-NH(2) (1), by substituting each amino acid in turn with its homologue. The ability to bind mu-opioid recept... |
|
|
Catalysis of 3-pyrrolidinecarboxylic acid and related pyrrolidine derivatives in enantioselective anti-Mannich-type reactions: importance of the 3-acid group on pyrrolidine for stereocontrol.
J. Am. Chem. Soc. 130(3) , 875-86, (2008) The development of enantioselective anti-selective Mannich-type reactions of aldehydes and ketones with imines catalyzed by 3-pyrrolidinecarboxylic acid and related pyrrolidine derivatives is reported in detail. Both (3R,5R)-5-methyl-3-pyrrolidinecarboxylic a... |
|
|
Non-hydrogen-bonded secondary structure in beta-peptides: evidence from circular dichroism of (S)-pyrrolidine-3-carboxylic acid oligomers and (S)-nipecotic acid oligomers.
Org. Lett. 1(11) , 1717-20, (1999) [formula: see text] Homooligomers of beta-amino acids (S)-3-pyrrolidine-3-carboxylic acid (PCA) and (S)-nipecotic acid (Nip) were studied by circular dichroism (CD) in methanol. In each series, a profound change in the far-UV CD spectrum was observed from mon... |
|
|
Constrained beta-proline analogues in organocatalytic aldol reactions: the influence of acid geometry.
J. Org. Chem. 74(14) , 5041-8, (2009) 7-Azabicyclo[2.2.1]heptane-2-carboxylic acid 11 was prepared in enantiopure form, and its catalytic potential in the direct aldol reaction between acetone and 4-nitrobenzaldehyde was assessed. The bicyclic system was found to be more selective than its monocy... |
|
|
Inhibitors of metalloendopeptidase EC 3.4.24.15 and EC 3.4.24.16 stabilized against proteolysis by the incorporation of beta-amino acids.
Biochemistry 41(35) , 10819-26, (2002) The enzyme EC 3.4.24.15 (EP 24.15) is a zinc metalloendopeptidase whose precise function in vivo remains unknown but is thought to participate in the regulated metabolism of a number of specific neuropeptides. The lack of stable and selective inhibitors has h... |
|
|
Antinociception by a peripherally administered novel endomorphin-1 analogue containing beta-proline.
Eur. J. Pharmacol. 469(1-3) , 89-95, (2003) We previously described a novel endomorphin-1 analogue (Tyr-L-beta-Pro-Trp-Phe-NH(2); Endo1-beta-Pro) more resistant to enzymatic hydrolysis than endomorphin-1 that acts as a mu-opioid receptor agonist. In this study we report that Endo1-beta-Pro, s.c. inject... |
|
|
Si-free enolate Claisen rearrangements of enamido substrates.
Org. Biomol. Chem. 10(7) , 1406-10, (2012) α-Alkyl β-amino esters are available in high diastereoselectivity through a silicon-free Claisen enolate [3,3]-sigmatropic rearrangement of enamide esters. Optimisation studies have probed the crucial role of the initial enolisation and the nature of the enam... |
|
|
A stereoselective cyclization strategy for the preparation of γ-lactams and their use in the synthesis of α-methyl-β-proline.
J. Org. Chem. 77(23) , 10925-30, (2012) A straightforward stereoselective and enantiodivergent cyclization strategy for the construction of γ-lactams is described. The cyclization strategy makes use of chiral malonic esters prepared from enantiomerically enriched monoesters of disubstituted malonic... |