3,4,5-三甲氧基苯甲酸
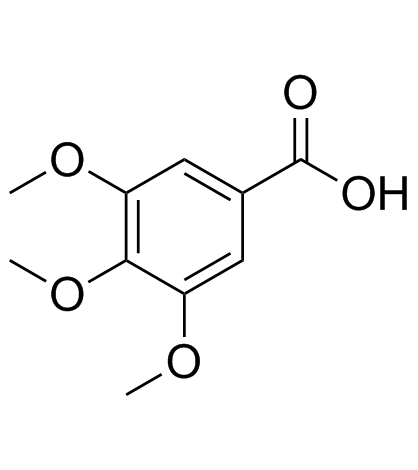
3,4,5-三甲氧基苯甲酸结构式

|
常用名 | 3,4,5-三甲氧基苯甲酸 | 英文名 | Trimethylgallic acid |
|---|---|---|---|---|
| CAS号 | 118-41-2 | 分子量 | 212.199 | |
| 密度 | 1.2±0.1 g/cm3 | 沸点 | 376.3±0.0 °C at 760 mmHg | |
| 分子式 | C10H12O5 | 熔点 | 168-171 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 128.8±20.0 °C |
|
Structure-Activity Relationships of Antimicrobial Gallic Acid Derivatives from Pomegranate and Acacia Fruit Extracts against Potato Bacterial Wilt Pathogen.
Chem. Biodivers. 12 , 955-62, (2015) Bacterial wilts of potato, tomato, pepper, and or eggplant caused by Ralstonia solanacearum are among the most serious plant diseases worldwide. In this study, the issue of developing bactericidal agents from natural sources against R. solanacearum derived fr... |
|
|
Relation between lipophilicity of alkyl gallates and antifungal activity against yeasts and filamentous fungi.
Bioorg. Med. Chem. Lett. 19 , 1793-6, (2009) The antifungal activity of a complete series of 15 n-alkyl gallates and six analogues acting against a representative panel of opportunistic pathogenic fungi was studied in order to analyze their role in: the importance of the fungi tested, the importance of ... |
|
|
(-) Epigallocatechin-3-gallate attenuates reserpine-induced orofacial dyskinesia and oxidative stress in rat striatum.
Pharmacol. Biochem. Behav. 131 , 71-6, (2015) Reserpine-induced orofacial dyskinesia (OD) has been used for decades as an animal model for human tardive dyskinesia (TD) because both of them have pathophysiology strongly associated with striatal oxidative stress. Green tea catechins, especially (-) epigal... |
|
|
Proton dissociation is important to understanding structure-activity relationships of gallic acid antioxidants.
Bioorg. Med. Chem. Lett. 16 , 4095-8, (2006) Gallic acid derivatives (GADs) can efficiently scavenge free radicals, which is partially responsible for their neuroprotective effects. As GADs tend to deprotonate to give birth to GAD anions, which has big influence on the radical-scavenging behaviors of GA... |
|
|
(+)-rumphiin and polyalthurea, new compounds from the stems of Polyalthia rumphii.
Nat. Prod. Commun. 8(10) , 1427-9, (2013) Two new compounds, (+)-rumphiin (3) and polyalthurea (7), together with seven known ones, 3,4,5-trimethoxy benzoic acid (1), (-)-seselinone (2), cannabisin D (4), allantoin (5), oxostephanine (6) and a mixture of beta-sitosterol (8) and stigmasterol (9) were ... |
|
|
Utilization of methoxylated benzoates and formation of intermediates by Desulfotomaculum thermobenzoicum in the presence or absence of sulfate.
Arch. Microbiol. 157(3) , 209-12, (1992) Desulfotomaculum thermobenzoicum strain TSB (DSM 6193) was found to utilize some methoxylated benzoates as carbon and energy source with or without sulfate. 3- or 4-Methoxybenzoate, vanillate (4-hydroxy-3-methoxybenzoate), syringate (3,5-dimethoxy-4-hydroxybe... |
|
|
Atropa belladonna hairy roots: orchestration of concurrent oxidation and reduction reactions for biotransformation of carbonyl compounds.
Appl. Biochem. Biotechnol. 166(6) , 1401-8, (2012) The biotransformation potential of a selected Atropa belladonna hairy root clone (AB-09) had been evaluated with regard to three different aromatic carbonyl compounds, i.e., 3,4,5-trimethoxybenzaldehyde (1), 3,4,5-trimethoxyacetophenone (2), and 3,4,5-trimeth... |
|
|
Aqueous chlorination of the antibacterial agent trimethoprim: reaction kinetics and pathways.
Water Res. 41(3) , 647-55, (2007) Trimethoprim (TMP), one of the antibacterials most frequently detected in municipal wastewaters and surface waters, reacts readily with free available chlorine (i.e., HOCl) at pH values between 3 and 9 (e.g., the pH-dependent apparent second-order rate consta... |
|
|
Production of methanol from aromatic acids by Pseudomonas putida.
J. Bacteriol. 142(3) , 916-24, (1980) When grown at the expense of 3,4,5-trimethoxybenzoic acid, a strain of Pseudomonas putida oxidized this compound and also 3,5-dimethoxy-4-hydroxybenzoic (syringic) and 3,4-dihydroxy-5-methoxybenzoic (3-O-methylgallic) acids; but other hydroxy- or methoxy-benz... |
|
|
[Synthesis and the study of the effect of new 3,4,5-trimethoxybenzoic acid derivatives on the central nervous system].
Acta Pol. Pharm. 42(2) , 117-22, (1985)
|