N-(2-氨基乙基)-4-氯苯甲酰胺盐酸盐
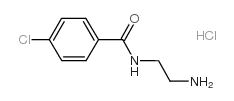
N-(2-氨基乙基)-4-氯苯甲酰胺盐酸盐结构式

|
常用名 | N-(2-氨基乙基)-4-氯苯甲酰胺盐酸盐 | 英文名 | Ro 16-6491 |
|---|---|---|---|---|
| CAS号 | 94319-79-6 | 分子量 | 235.11000 | |
| 密度 | N/A | 沸点 | 404.2ºC at 760 mmHg | |
| 分子式 | C9H12Cl2N2O | 熔点 | 209-210ºC | |
| MSDS | 中文版 美版 | 闪点 | 198.2ºC |
|
The effects of phenelzine and other monoamine oxidase inhibitor antidepressants on brain and liver I2 imidazoline-preferring receptors.
Br. J. Pharmacol. 114(4) , 837-45, (1995) 1. The binding of [3H]-idazoxan in the presence of 10(-6) M (-)-adrenaline was used to quantitate I2 imidazoline-preferring receptors in the rat brain and liver after chronic treatment with various irreversible and reversible monoamine oxidase (MAO) inhibitor... |
|
|
Overview of the present state of MAO inhibitors.
J. Neural Transm. Suppl. 23 , 103-19, (1987) In this paper an overview of the present state of monoamine oxidase inhibitors (MAOIs) is presented. The irreversible inhibitors are firstly considered. They have been divided into four chemical types: substituted hydrazine, cyclopropylamine, propargylamine a... |
|
|
Synthesis and characterization of [125I]N-(2-aminoethyl)-4-iodobenzamide as a selective monoamine oxidase B inhibitor.
Nucl. Med. Biol. 22(5) , 617-23, (1995) We described the radiosynthesis of an analog of Ro 16-6491, [125I]N-(2-aminoethyl)-4-iodobenzamide, for SPECT exploration of the monoamine oxidase B (MAO-B) in human brain. The radiolabelling was carried out by nucleophilic exchange of the brominated precurso... |
|
|
Aromatic L-amino acid decarboxylase (AAAD) activity in rhesus macaque striatum after MAO-B inhibition by Ro 16-6491.
Synapse 56(1) , 54-6, (2005)
|
|
|
Interactions of the novel inhibitors of MAO-B Ro 19-6327 and Ro 16-6491 with the active site of the enzyme.
Pharmacol. Res. Commun. 20 , 51, (1988)
|
|
|
[3H]Ro 16-6491, a selective probe for affinity labelling of monoamine oxidase type B in human brain and platelet membranes.
J. Neurochem. 50 , 1037, (1988) [3H]Ro 16-6491 [N-(2-aminoethyl)-p-chlorobenzamide HCl], a reversible "mechanism-based" inhibitor of monoamine oxidase (MAO) type B, binds selectively and with high affinity to the active site of MAO-B in brain and platelet membranes. Under normal conditions,... |
|
|
Short-acting novel MAO inhibitors: in vitro evidence for the reversibility of MAO inhibition by moclobemide and Ro 16-6491.
Naunyn Schmiedebergs Arch. Pharmacol. 335 , 12-6491, (1987) The inhibition of monoamine oxidase (MAO) in rat liver and brain by the short-acting MAO-A inhibitors moclobemide (Ro 11-1163 = p-chloro-N-[2-morpholinoethyl]benzamide) and brofaremine and by the short-acting MAO-B inhibitors Ro 16-6491 (N-[2-aminoethyl]-p-ch... |
|
|
Neural progenitor cells are protected against MPTP by MAO-B inhibitors.
Neurotoxicology 29(6) , 1141-6, (2008) Neurotoxic effects of MPTP on the nigrostriatal dopaminergic system are thought to be initiated by 1-methyl-4-phenylpyridinium (MPP+), a metabolite formed by the monoamine oxidase (MAO)-B-mediated oxidation of MPTP. We previously reported that the administrat... |
|
|
Kinetics of inhibition of MAO-B by N-(2-aminoethyl)-p-chlorobenzamide (Ro 16-6491) and analogues.
J. Neural Transm. Suppl. 41 , 307-11, (1994) Ro 16-6491 is known to be a potent reversible inhibitor of human brain MAO-B. This compound and several analogues were tested for their effect on bovine liver MAO-B. It was found that in compounds where the amide bond of Ro 16-6491 was replaced by an ester bo... |
|
|
Ro 16-6491: a new reversible and highly selective MAO-B inhibitor protects mice from the dopaminergic neurotoxicity of MPTP.
Adv. Neurol. 45 , 175-8, (1987)
|