喹哪啶酸
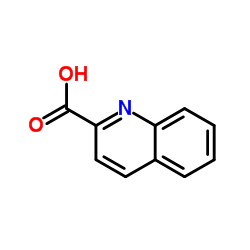
喹哪啶酸结构式

|
常用名 | 喹哪啶酸 | 英文名 | Quinoline-2-carboxylic acid |
|---|---|---|---|---|
| CAS号 | 93-10-7 | 分子量 | 173.168 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 348.7±17.0 °C at 760 mmHg | |
| 分子式 | C10H7NO2 | 熔点 | 156-158 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 164.7±20.9 °C |
|
Biosynthesis of 8-hydroxyquinoline-2-carboxylic acid, an iron chelator from the gut of the lepidopteran Spodoptera littoralis.
Org. Biomol. Chem. 13(1) , 178-84, (2014) In the regurgitate (foregut content) of Spodoptera larvae we found high concentrations (0.5-5 mM) of 8-hydroxyquinoline-2-carboxylic acid (8-HQA). In a survey of different lepidopteran species, this compound was only detected in species belonging to the famil... |
|
|
Hydrogen-bonded frameworks of bis(2-carboxypyridinium) hexafluorosilicate and bis(2-carboxyquinolinium) hexafluorosilicate dihydrate.
Acta Crystallogr. C 63(Pt 9) , o530-4, (2007) In bis(2-carboxypyridinium) hexafluorosilicate, 2C(6)H(6)NO(2)+.SiF6(2-), (I), and bis(2-carboxyquinolinium) hexafluorosilicate dihydrate, 2C(10)H(8)NO(2)+.SiF6(2-).2H2O, (II), the Si atoms of the anions reside on crystallographic centres of inversion. Primar... |
|
|
How thiostrepton was made in the laboratory.
Angew. Chem. Int. Ed. Engl. 51(50) , 12414-36, (2012) Thiostrepton, a powerful antibiotic belonging to the thiopeptide class, was synthesized in the laboratory for the first time in 2004 through an arduous campaign involving novel strategies and tactics, scenic detours, and unexpected roadblocks. In this Review ... |
|
|
Insights into quinaldic acid moiety formation in thiostrepton biosynthesis facilitating fluorinated thiopeptide generation.
Chem. Biol. 19(4) , 443-8, (2012) Thiostrepton (TSR), often referred as to a parent compound in the thiopeptide family, is a bimacrocyclic member that features a quinaldic acid (QA) moiety-containing side ring appended to the characteristic core system. QA biosynthesis requires an unusual rin... |
|
|
Synthesis, characterization and crystal structure of novel mononuclear peroxotungsten(VI) complexes. Insulinomimetic activity of W(VI) and Nb(V) peroxo complexes.
J. Inorg. Biochem. 103(5) , 859-68, (2009) Two new mononuclear peroxo complexes of tungsten of the formula (gu)(2)[WO(2)(O(2))(2)] (1) and (gu)[WO(O(2))(2)(quin-2-c)] (2a) (where gu(+)=guanidinium ion, CN(3)H(6)(+) and quin-2-c=quinoline-2-carboxylate ion) have been synthesized and characterized by el... |
|
|
The role of dietary ergothioneine in the development of diabetes mellitus.
Med. Hypotheses 9(2) , 207-13, (1982) Ergothioneine is believed not to be synthesized by man but it accumulates to high concentrations in some mammalian cells as a result of dietary intake. Ergothioneine is known to chelate divalent metal ions with high affinity. Other substances which are potent... |
|
|
Transcriptional response pathways in a yeast strain sensitive to saframycin a and a more potent analog: evidence for a common basis of activity.
Chem. Biol. 9(5) , 607-18, (2002) Saframycin A (SafA) is a natural product that inhibits human cancer cell proliferation. Its synthetic analog, QAD, is a more potent inhibitor of these cells. SafA does not affect wild-type yeast, but it does inhibit growth of the strain CCY333 (DeltaPDR1/PDR3... |
|
|
Microbial metabolism of quinoline and related compounds. XX. Quinaldic acid 4-oxidoreductase from Pseudomonas sp. AK-2 compared to other procaryotic molybdenum-containing hydroxylases.
Biol. Chem. Hoppe-Seyler 374(11) , 1037-46, (1993) Quinaldic acid 4-oxidoreductase from Pseudomonas sp. AK-2 catalyses the hydroxylation of quinoline 2-carboxylic (quinaldic acid) to 4-hydroxyquinoline 2-carboxylic acid (kynurenic acid) with concomitant reduction of a suitable electron acceptor. An analogous ... |
|
|
Inhibition of dopamine beta-hydroxylase by bidentate chelating agents.
Biochim. Biophys. Acta 1037(2) , 240-7, (1990) 1-2H-Phthalazine hydrazone (hydralazine; HYD), 2-1H-pyridinone hydrazone (2-hydrazinopyridine; HP), 2-quinoline-carboxylic acid (QCA), 1-isoquinolinecarboxylic acid (IQCA), 2,2'-bi-1H-imidazole (2,2'-biimidazole; BI), and 1H-imidazole-4-acetic acid (imidazole... |
|
|
4-Carboxyanilinium (2R,3R)-tartrate and a redetermination of the alpha-polymorph of 4-aminobenzoic acid.
Acta Crystallogr. C 63(Pt 9) , o514-7, (2007) In the title compounds, 4-carboxyanilinium (2R,3R)-tartrate, C(7)H(8)NO(2)+.C(4)H(5)O(6)-, (I), and 4-aminobenzoic acid, C(7)H(7)NO(2), (II), the carboxyl planes of the 4-carboxyanilinium cations/4-aminobenzoic acid are twisted from the aromatic plane. In (I)... |