L-(+)-苏糖
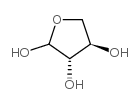
L-(+)-苏糖结构式

|
常用名 | L-(+)-苏糖 | 英文名 | L-(+)-threose |
|---|---|---|---|---|
| CAS号 | 95-44-3 | 分子量 | 120.10400 | |
| 密度 | 1.698g/cm3 | 沸点 | 290.6ºC at 760mmHg | |
| 分子式 | C4H8O4 | 熔点 | 162-163℃ | |
| MSDS | 美版 | 闪点 | 129.5ºC |
|
Synthesis of threose nucleic acid (TNA) triphosphates and oligonucleotides by polymerase-mediated primer extension.
Curr. Protoc. Nucleic Acid Chem. Chapter 4 , Unit 4.54, (2013) This unit describes the chemical synthesis of α-L-threofuranosyl nucleic acid (TNA) triphosphates for thymidine (T), guanosine (G), cytidine (C), and the diaminopurine (D) analog of adenosine and their incorporation into TNA oligonucleotides by enzyme-mediate... |
|
|
The silicate-mediated formose reaction: bottom-up synthesis of sugar silicates.
Science 327(5968) , 984-6, (2010) Understanding the mechanism of sugar formation and stabilization is important for constraining theories on the abiotic origin of complex biomolecules. Although previous studies have produced sugars from small molecules through the formose and related reaction... |
|
|
Formation of furan and methylfuran from ascorbic acid in model systems and food.
Food Addit. Contam. 24 Suppl 1 , 122-35, (2007) Previous model studies have suggested ascorbic acid as one of the major sources of furan, a possibly hazardous compound found in thermally processed foods (e.g. canned products, jars). The study showed that about 2 mmol mol(-1) furan was obtained when dry-hea... |
|
|
Nonenzymatic oligomerization of RNA by TNA templates.
Org. Lett. 8(25) , 5809-11, (2006) Cytosine TNA promotes nonenzymatic, template-directed oligomerization of complementary activated rGMP, leading to selective and efficient formation of RNA products. This process models "genetic takeover" of a pre-RNA by RNA. [reaction: see text] |
|
|
Kinetic characterization and comparison of various protein crosslinking reagents for matrix modification.
J. Mater. Sci. Mater. Med. 21(4) , 1175-81, (2010) We have characterized the relative efficacies of a number of protein crosslinking agents that have the potential for use in the crosslinking of proteinaceous matrices both in vitro and in vivo. The crosslinkers tested were; L: -threose (LT), Genipin (GP), Met... |
|
|
Efficient asymmetric organocatalytic formation of erythrose and threose under aqueous conditions.
Chem. Commun. (Camb.) 46(26) , 4776-8, (2010) Esters of proteinogenic amino acids efficiently catalyse the formation of erythrose and threose under aqueous conditions in the highest yields and enantioselectivities yet reported. Remarkably while esters of (L)-proline yield (L)-carbohydrates, esters of (L)... |
|
|
Synthesis and evaluation of amino-threoses in D- and L-series: are five membered ring amino-sugars more potent glycosidase inhibitors than the six membered ones?
Bioorg. Med. Chem. 15(12) , 4125-35, (2007) Cyclic D- and L-4-aminothreose were synthesised from ethyl D- and L-tartrate, respectively. D-aminothreose was a potent inhibitor of alpha-glucosidase and of alpha-mannosidase. From the glycosidase inhibition potencies of the four 4-amino-4-deoxy-tetroses, th... |
|
|
On the basics of carbohydrate-metal chemistry: complexes of palladium(II) with hydroxyaldehyde and -ketone hydrates.
Carbohydr. Res. 342(11) , 1419-26, (2007) In this work, we present novel complexes of hydroxyaldehydes and ketones with palladium(II). The compounds are studied by two-dimensional NMR spectroscopy in solution (COSY, HMQC, HMBC). Glycolaldehyde, d-glyceraldehyde, glyoxal and 2,4-O-ethylidene-d-erythro... |
|
|
Beneficial effect of internal hydrogen bonding interactions on the beta-fragmentation of primary alkoxyl radicals. Two-step conversion of D-xylo- and D-ribofuranoses into L-threose and D-erythrose, respectively.
J. Org. Chem. 72(22) , 8196-201, (2007) Primary alkoxyl free radicals were generated from their readily synthesized N-phthalimido derivatives under reductive conditions. Primary alkoxyl radicals derived from their corresponding xylo- and ribofuranose derivatives underwent, exclusively, an unusual b... |
|
|
Nondestructive fluorescence-based quantification of threose-induced collagen cross-linking in bovine articular cartilage.
J. Biomed. Opt. 17(9) , 97003, (2012) Extensive collagen cross-linking affects the mechanical competence of articular cartilage: it can make the cartilage stiffer and more brittle. The concentrations of the best known cross-links, pyridinoline and pentosidine, can be accurately determined by dest... |