2Z,6Z-Farnesol
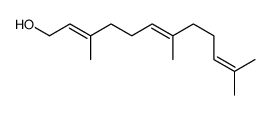
2Z,6Z-Farnesol结构式

|
常用名 | 2Z,6Z-Farnesol | 英文名 | 2Z,6Z-Farnesol |
|---|---|---|---|---|
| CAS号 | 16106-95-9 | 分子量 | 222.36600 | |
| 密度 | 0.875g/cm3 | 沸点 | 283.4ºC at 760 mmHg | |
| 分子式 | C15H26O | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 112.5ºC |
|
Ras signalling and apoptosis.
Curr. Opin. Genet. Dev. 8 , 49-54, (1998) Activated Ras proteins have either positive or negative effects on the regulation of apoptosis depending on cell type and other factors. In part, this is due to the ability of Ras to control directly multiple effector pathways, including PI3-kinase, which pro... |
|
|
Synthesis of farnesol isomers via a modified Wittig procedure.
Org. Lett. 7 , 4803-4806, (2005) [structure: see text] The four olefin stereoisomers of farnesol have been synthesized from readily available nerylacetone or commercial geranylacetone. A new variation on the use of beta-oxido ylides favored the (2Z)-stereoisomers, whereas the (2E)-isomers we... |
|
|
Quorum sensing in Candida albicans: probing farnesol's mode of action with 40 natural and synthetic farnesol analogs.
Chem. Biol. 10 , 743-50, (2003) The dimorphic fungus Candida albicans produces extracellular farnesol (3,7,11-trimethyl-2,6,10-dodecatriene-1-ol) which acts as a quorum-sensing molecule (QSM) to suppress filamentation. Of four possible geometric isomers of farnesol, only the E,E isomer poss... |
|
|
The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action.
FEMS Microbiol. Lett. 237 , 325-331, (2004) The study was made of the antibacterial effects of three terpene alcohols on Staphylococcus aureus, focusing on the leakage of K+ ions and toxicity over time. The leakage of K+ ions was monitored continuously with a K+-electrode. Our results suggested that th... |
|
|
Synthesis and biological evaluation of the geometric farnesylated analogues of the a-factor mating peptide of Saccharomyces cerevisiae.
J. Org. Chem. 65 , 8552-8563, (2000) The a-factor of Saccharomyces cerevisiae is a dodecapeptide pheromone (YIIKGVFWDPAC(Farnesyl)-OCH(3), 1), in which post-translational modification with a farnesyl isoprenoid and carboxymethyl group is required for full biological activity. This peptide has be... |
|
|
Stereochemistry-dependent inhibition of RAS farnesylation by farnesyl phosphonic acids.
Lipids 33 , 39-46, (1998) This investigation compares the effects of three farnesyl pyrophosphate analogs on selected aspects of isoprenoid metabolism. E,E-alpha-Hydroxyfarnesylphosphonate was prepared by an improved variation on a literature synthesis, which also gave access to the n... |