断马钱子苷
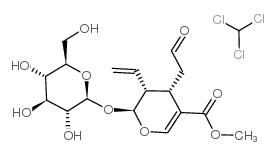
断马钱子苷结构式

|
常用名 | 断马钱子苷 | 英文名 | Secologanin |
|---|---|---|---|---|
| CAS号 | 19351-63-4 | 分子量 | 507.74400 | |
| 密度 | 1.42g/cm3 | 沸点 | 595.5ºC at 760mmHg | |
| 分子式 | C17H24O10 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | 212.2ºC |
|
The leaf epidermome of Catharanthus roseus reveals its biochemical specialization.
Plant Cell 20(3) , 524-42, (2008) Catharanthus roseus is the sole commercial source of the monoterpenoid indole alkaloids (MIAs), vindoline and catharanthine, components of the commercially important anticancer dimers, vinblastine and vincristine. Carborundum abrasion technique was used to ex... |
|
|
2,4-D and alkaloid accumulation in periwinkle cell suspensions.
Biochimie 76(5) , 410-6, (1994) Omission of 2,4-D from culture medium during one subculture of Catharanthus roseus cells, strain C20, resulted in an increased alkaloid accumulation, without effect on growth. Alkaloid accumulation, rather than growth, seemed to be more sensitive to 2,4-D. 2,... |
|
|
Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase.
Plant J. 24 , 797, (2000) The molecular characterization of CYP72A1 from Catharanthus roseus (Madagascar periwinkle) was described nearly a decade ago, but the enzyme function remained unknown. We now show by in situ hybridization and immunohistochemistry that the expression in immatu... |
|
|
Strategies for engineering plant natural products: the iridoid-derived monoterpene indole alkaloids of Catharanthus roseus.
Meth. Enzymol. 515 , 189-206, (2012) The manipulation of pathways to make unnatural variants of natural compounds, a process often termed combinatorial biosynthesis, has been robustly successful in prokaryotic systems. The development of approaches to generate new-to-nature compounds from plant-... |
|
|
Secologanin synthase which catalyzes the oxidative cleavage of loganin into secologanin is a cytochrome P450.
Phytochemistry 53(1) , 7-12, (2000) Secologanin synthase, an enzyme catalyzing the oxidative cleavage of the cyclopentane ring in loganin to form secologanin, was detected in microsomal preparations from cell suspension cultures of Lonicera japonica. The reaction required NADPH and molecular ox... |
|
|
Investigation of Pictet-Spengler type reactions of secologanin with histamine and its benzyl derivative.
J. Nat. Prod. 65(5) , 649-55, (2002) The reaction of secologanin (1) (mainly in its tetraacetylated form 1a) with histamine (2) and its benzyl derivative (2b) was investigated. With the benzylated amine (2b), the main product was the normal, tetraacetylated benzyl derivative of histeloside havin... |
|
|
Two chromone-secoiridoid glycosides and three indole alkaloid glycosides from Neonauclea sessilifolia.
Phytochemistry 62(3) , 359-69, (2003) From the dried roots of Neonauclea sessilifolia, two new chromone-secoiridoid glycosides, sessilifoside and 7"-O-beta-D-glucopyranosylsessilifoside, and three novel indole alkaloid glycosides, neonaucleosides A, B, and C, were isolated along with the main kno... |
|
|
Investigation of the coupling reaction of tetraacetylsecologanin with oxotryptamine and its derivative.
J. Nat. Prod. 64(8) , 1032-9, (2001) The coupling reaction of tetraacetylsecologanin with 2,3-dihydro-2-oxotryptamine and its N(b)-benzyl derivative was investigated. With the benzylated amine, the reaction was stopped at the tetracyclic ester level, and with the unsubstituted amine it was immed... |
|
|
Regio- and stereoselectivity in the coupling reaction of secologanin with dopamine derivatives.
J. Nat. Prod. 64(3) , 332-40, (2001) The coupling reaction of tetraacetylsecologanin with dopamine and its N-benzyl derivative was investigated. In both series, stereoisomers at C-1, as well as regioisomer normal and neo compounds, were formed. Moreover, the N-unsubstituted products were partial... |
|
|
Improved expression of His(6)-tagged strictosidine synthase cDNA for chemo-enzymatic alkaloid diversification.
Chem. Biodivers. 7(4) , 860-70, (2010) Strictosidine synthase (STR1) catalyzes the stereoselective formation of 3alpha(S)-strictosidine from tryptamine and secologanin. Strictosidine is the key intermediate in the biosynthesis of 2,000 plant monoterpenoid indole alkaloids, and it is a key precurso... |