2-氯苯并噻唑
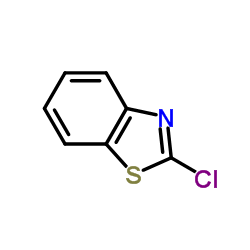
2-氯苯并噻唑结构式

|
常用名 | 2-氯苯并噻唑 | 英文名 | 2-Chlorobenzothiazole |
|---|---|---|---|---|
| CAS号 | 615-20-3 | 分子量 | 169.631 | |
| 密度 | 1.4±0.1 g/cm3 | 沸点 | 248.0±9.0 °C at 760 mmHg | |
| 分子式 | C7H4ClNS | 熔点 | 21-23 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 103.8±18.7 °C |
|
Synthesis and antileishmanial activity of (1,3-benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives.
Eur. J. Med. Chem. 39(8) , 685-90, (2004) (1,3-Benzothiazol-2-yl) amino-9-(10H)-acridinone derivatives were synthesized via a procedure based on the Ullman reaction and were assessed for their in vitro antileishmanial and anti-HIV activities. Two derivatives, 4-(6-nitro-benzothiazol-2-ylamino)-10H-ac... |
|
|
Synthesis of new thienopyrimidobenzothiazoles and thienopyrimidobenzoxazoles with analgesic and antiinflammatory properties. Russo F, et al.
Eur. J. Med. Chem. 29(7) , 569-578, (1994)
|
|
|
Protecting group-free concise synthesis of (RS)/(S)-lubeluzole.
Org. Lett. 15(6) , 1158-61, (2013) Three new, concise, and protecting group-free synthetic routes for (RS)- and (S)-lubeluzole are reported in higher (46-62%) overall yields compared to the reported procedures (6-35%). The key steps involve C-N bond formation via epoxide aminolysis and nucleop... |