| Description |
Palmatine hydroxide is an orally active and irreversible indoleamine 2,3-dioxygenase 1 (IDO-1) inhibitor with IC50s of 3 μM and 157μM against HEK 293-hIDO-1 and rhIDO-1, respectively. Palmatine hydroxide can also inhibit West Nile virus (WNV) NS2B-NS3 protease in an uncompetitive manner with an IC50 of 96 μM. Palmatine hydroxide shows anti-cancer, anti-oxidation, anti-inflammatory, neuroprotection, antibacterial, anti-viral activities[1][2][3][4][5].
|
| Related Catalog |
|
| Target |
IDO-1:3 μM (IC50, HEK 293-hIDO-1)
IDO-1:157 μM (IC50, rhIDO-1)
WNV NS2B-NS3:96 μM (IC50)
|
| In Vitro |
Palmatine (0-100 μM; 42 h) suppresses WNV with an EC50 value of 3.6 μM, and reduce the viral titers of DENV-2 and YFV with EC50 values of 26.4 μM and 7.3 μM, respectively[3]. Palmatine (0-1128 μM; 24-72 h) inhibits colon cancer cell proliferation[5]. Palmatine (0-704 μM; 24 h) reduces AURKA protein levels, induces G2/M phase arrest, and induces apoptosis in colon cancer cells via the mitochondrial associated pathway[5]. Cell Proliferation Assay[5] Cell Line: HCT-116, SW480, HT-29 Concentration: 0, 88, 176, 352, and 704 μM (HCT-116, SW480); 0, 141, 282, 564, and 1128 μM (HT-29) Incubation Time: 24, 48 and 72 h Result: Decreased cell viability in a dose-dependent manner. Western Blot Analysis[5] Cell Line: HCT-116, SW480, HT-29 Concentration: 100 nM for HCT-116, 500 nM for SW480 and HT-29 Incubation Time: 24, 48 and 72 h Result: Promoted the expression of apoptosis markers such as P53 / P73, Caspase3, and Caspase9. Reduced AURKA protein levels. Increased cyt. c in the cytoplasm while reduced Bcl2 and Bcl-xl in a dose-dependent manner. Cell Cycle Analysis[5] Cell Line: HCT-116, SW480 Concentration: 88, 176, 352 and 704 μM Incubation Time: 24, 48 and 72 h Result: Induced G2/M phase arrest in a dose-dependent manner. Apoptosis Analysis[5] Cell Line: HCT-116, SW480 Concentration: 88, 176, 352 and 704 μM Incubation Time: 24, 48 and 72 h Result: Induced apoptosis in a dose-dependent manner.
|
| In Vivo |
Palmatine (50 or 100 mg/kg; p.o.; daily for 7 days) ameliorates DSS (dextran sulfate sodium)-induced colitis and prevents infiltration of inflammatory cells[1]. Palmatine (0-200 mg/kg; i.p.; once) attenuates D-galactosamine/Lipopolysaccharides (HY-D1056)-induced fulminant hepatic failure in mice[2]. Palmatine (0-1 mg/kg; i.p.; 10 days) shows memory-enhancing activity in mice[4]. Palmatine (33.75-135 mg/kg; p.o.; daily for 26 days) can effectively inhibit the growth of HCT-116 xenografts in mice[5]. Animal Model: DSS- induced Colitis BALB/c mice model (8-week-old)[1] Dosage: 50 or 100 mg/kg Administration: Orally, daily, for 7 days Result: Ameliorated DSS-induced colitis and prevented infiltration of inflammatory cells; remarkably extended the colon length; significantly suppressed the colonic MPO activity. Decreased the levels of colonic inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL-6, IL-4 and IL-10); Protected mucosal integrity by modulating TJs protein and apoptosis proteins; Restored DSS-induced decreases of TJ protein ZO-1, ZO-2 and claudin-1; Reduced Bax expression and enhanced Bcl-2 expression at the dose of 100 mg/kg, prevented epithelial apoptosis and improved intestinal integrity. Prevented DSS-induced changes of gut microbiota in colitis mice. Animal Model: Male ICR mice (20–22 g), D-galactosamine/lipopolysaccharide (GalN/LPS)-induced fulminant hepatic failure model[2] Dosage: 25, 50, 100, or 200 mg/kg Administration: Intraperitoneal injection, 1 h before the GalN/LPS treatment Result: Attenuated the mortality and serum aminotransferase activities increased by GalN/LPS. Prevented the increase of serum TNF-α and augmented that of serum IL-10. Decreased the TNF-a mRNA expression and increased the IL-10 mRNA expression. Attenuated the apoptosis of hepatocytes. Animal Model: Swiss young male albino mice, with Scopolamine (HY-N0296)- and diazepam-induced amnesia model[4] Dosage: 0.1, 0.5, 1 mg/kg Administration: Intraperitoneal injection, 10 days Result: Significantly improved learning and memory of mice at 0.5 and 1 mg/kg and did not show any significant effect on locomotor activity of the mice. Significantly reversed scopolamine- and diazepam-induced amnesia in mice. Significantly reduced brain acetylcholinesterase activity of mice. Animal Model: BALB/c-nude mice, HCT-116 xenograft model[5] Dosage: 33.75, 67.5 and 135 mg/kg Administration: Oral administration, once a day for 26 days Result: The tumor volume and weight of the treatment group were significantly reduced.
|
| References |
[1]. Zhang XJ, et al. Palmatine ameliorated murine colitis by suppressing tryptophan metabolism and regulating gut microbiota.Pharmacol Res. 2018 Nov;137:34-46. [2]. Lee WC, et al. Palmatine attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Food Chem Toxicol. 2010 Jan;48(1):222-8. [3]. Jia F, et al. Identification of palmatine as an inhibitor of West Nile virus. Arch Virol. 2010 Aug;155(8):1325-9. [4]. Dhingra D, et al. Memory-enhancing activity of palmatine in mice using elevated plus maze and morris water maze. Adv Pharmacol Sci. 2012;2012:357368. [5]. Liu X, et al. Palmatine induces G2/M phase arrest and mitochondrial-associated pathway apoptosis in colon cancer cells by targeting AURKA. Biochem Pharmacol. 2020 May;175:113933. [6]. Long J, et al. Palmatine: A review of its pharmacology, toxicity and pharmacokinetics. Biochimie. 2019 Jul;162:176-184.
|
![2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium,hydroxide Structure](https://www.chemsrc.com/caspic/201/131-04-4.png)
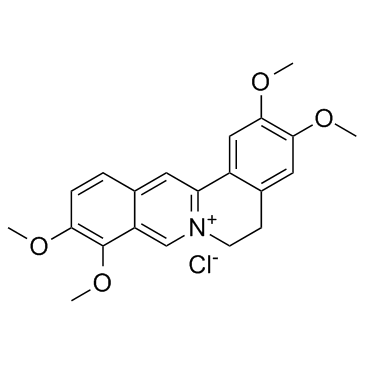 CAS#:10605-02-4
CAS#:10605-02-4 CAS#:63490-92-6
CAS#:63490-92-6