GDC-0941
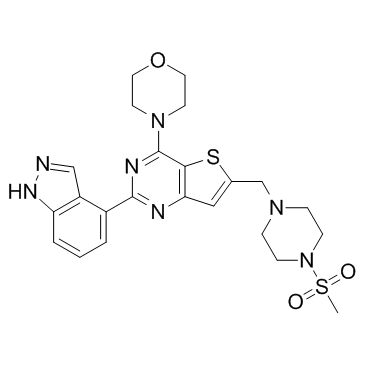
GDC-0941 structure
|
Common Name | GDC-0941 | ||
|---|---|---|---|---|
| CAS Number | 957054-30-7 | Molecular Weight | 513.636 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 687.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C23H27N7O3S2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 369.7±34.3 °C | |
Use of GDC-0941GDC-0941 (Pictilisib) is a potent inhibitor of PI3Kα/δ with an IC50 of 3 nM, with modest selectivity against p110β (11-fold) and p110γ (25-fold). |
| Name | pictrelisib |
|---|---|
| Synonym | More Synonyms |
| Description | GDC-0941 (Pictilisib) is a potent inhibitor of PI3Kα/δ with an IC50 of 3 nM, with modest selectivity against p110β (11-fold) and p110γ (25-fold). |
|---|---|
| Related Catalog | |
| Target |
p110α:3 nM (IC50) p110α-H1047R:3 nM (IC50) p110α-E545K:3 nM (IC50) p110δ:3 nM (IC50) p110β:33 nM (IC50) p110γ:75 nM (IC50) mTOR:0.58 μM (Ki) DNA-PK:1.23 μM (IC50) Autophagy |
| In Vitro | GDC-0941 and docetaxel reduce tumor cell viability by 80% or greater in the breast cancer cell lines than single-agent treatment. GDC-0941 inhibits Akt phosphorylation and downstream targets of Akt signaling such as pPRAS40 and pS6 in Hs578T1.2 (PI3Kα wild-type), MCF7-neo/HER2 (PI3Kα-mutant), and MX-1 (PTEN-null) tumor models. GDC-0941 decreases the time of docetaxel-induced mitotic arrest prior to apoptosis[1]. GDC-0941 shows a high efficacy of antitumor activity in two gefitinib-resistant non-small cell lung cancer (NSCLC) cell lines, A549 and H460. GDC-0941 is highly efficacious in combination with U0126 in inducing cell growth inhibition, G0-G1 arrest and cell apoptosis. H460 cells with activating mutations of PIK3CA are relatively more sensitive to GDC-0941 than A549 cells with wild-type PIK3CA[3]. GDC-0941 reduces PI3K pathway activity in both cell lines, illustrated by decreased pAK. GDC-0941 significantly reduces secreted VEGF detected in the medium after hypoxic/anoxic exposure in all cells[4]. |
| In Vivo | GDC-0941 (150 mg/kg, p.o.) leads to tumor stasis in MCF7-neo/HER2-bearing animals model. GDC-0941 and docetaxel result in tumor regressions during the treatment period leading to enhanced antitumor responses[1]. Tumours in the GDC-0941-treated mice show a marked non-linear shrinkage, and when the GDC-0941 treatment ceased, the tumours in the test cohort mice grow again[2]. GDC-0941 (25 or 50 mg/kg) reduces tumor growth and PI3K and HIF-1 pathway activity in eGFP-FTC133 tumor-bearing mice[4]. |
| Cell Assay | Cells are treated at EC50 concentrations of GDC-0941, docetaxel, or both for 4 or 24 hours and lysed in 1×Cell Extraction Buffer supplemented with protease inhibitors and Phosphatase Inhibitor Cocktails 1 and 2. Protein concentrations are determined using the Pierce BCA Protein Assay Kit. For immunoblots, equal amounts of protein are separated by electrophoresis through NuPAGE Bis-Tris 10% gradient gels, transferred onto polyvinylidene difluoride membranes using the Criterion system, and probed with monospecific primary antibodies. Specific antigen-antibody interactions are detected with IRDye 680 or IRDye 800 infrared secondary antibodies using a LI-COR imaging system. |
| Animal Admin | Female nu/nu mice are inoculated subcutaneously with MCF7-neo/HER2 or MX-1 breast cancer cells. When tumors reach a mean volume of 200 to 250 mm3, animals are size-matched and distributed into groups consisting of 10 animals per group. Docetaxel formulated in 3% EtOH, 97% saline is administered intravenously once weekly. GDC-0941, formulated in MCT (0.5% methylcellulose, 0.2% Tween-80) is dosed orally and daily. MAXF1162 is an HER2+/ER+/PR+ patient-derived breast cancer tumor xenograft model established by directly implanting tumors subcutaneously from patient to NMRI nu/nu mice. Tumor volume is calculated. Tumor sizes are recorded twice weekly over the course of a study. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 687.7±65.0 °C at 760 mmHg |
| Molecular Formula | C23H27N7O3S2 |
| Molecular Weight | 513.636 |
| Flash Point | 369.7±34.3 °C |
| Exact Mass | 513.161682 |
| PSA | 144.17000 |
| LogP | 2.04 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.753 |
| HS Code | 29350090 |
|---|
| Precursor 10 | |
|---|---|
| DownStream 0 | |
| 4-(2-(1H-indazol-4-yl)-6-((4-(methylsulfonyl)piperazin-1-yl)methyl)thieno[3,2-d]pyrimidin-4-yl)morpholine |
| pictrelisib |
| Thieno[3,2-d]pyrimidine, 2-(1H-indazol-4-yl)-6-[[4-(methylsulfonyl)-1-piperazinyl]methyl]-4-(4-morpholinyl)- |
| GDC0941 |
| GDC-941 |
| GDC 0941 |
| 2-(1H-Indazol-4-yl)-6-{[4-(methylsulfonyl)piperazin-1-yl]methyl}-4-(morpholin-4-yl)thieno[3,2-d]pyrimidin |
| 2-(1H-Indazol-4-yl)-6-{[4-(methylsulfonyl)-1-piperazinyl]methyl}-4-(4-morpholinyl)thieno[3,2-d]pyrimidine |
| Pictilisib |
| 2-(1H-Indazol-4-yl)-6-[[4-(methylsulfonyl)-1-piperazinyl]methyl]-4-(4-morpholinyl)thieno[3,2-d]pyrimidine |
| GDC-0941 |
| GDC-0941 bismesylate |
 CAS#:885618-33-7
CAS#:885618-33-7![4-(2-CHLORO-6-((4-(METHYLSULFONYL)PIPERAZIN-1-YL)METHYL)THIENO[3,2-D]PYRIMIDIN-4-YL)MORPHOLINE Structure](https://image.chemsrc.com/caspic/062/885675-66-1.png) CAS#:885675-66-1
CAS#:885675-66-1![4-(2-Chlorothieno[3,2-d]pyrimidin-4-yl)morpholine Structure](https://image.chemsrc.com/caspic/394/16234-15-4.png) CAS#:16234-15-4
CAS#:16234-15-4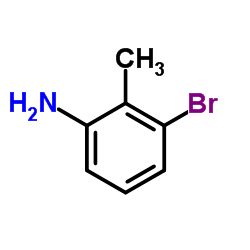 CAS#:55289-36-6
CAS#:55289-36-6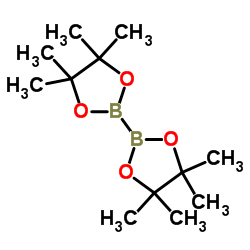 CAS#:73183-34-3
CAS#:73183-34-3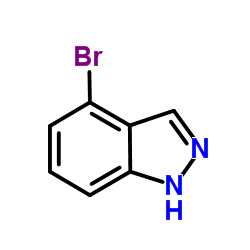 CAS#:186407-74-9
CAS#:186407-74-9![Thieno[3,2-d]pyrimidine-2,4-diol Structure](https://image.chemsrc.com/caspic/064/16233-51-5.png) CAS#:16233-51-5
CAS#:16233-51-5 CAS#:22288-78-4
CAS#:22288-78-4![2,4-Dichlorothieno[3,2-d]pyrimidine Structure](https://image.chemsrc.com/caspic/432/16234-14-3.png) CAS#:16234-14-3
CAS#:16234-14-3![2-Chloro-4-morpholinothieno[3,2-d]pyrimidine-6-carbaldehyde Structure](https://image.chemsrc.com/caspic/051/885618-31-5.png) CAS#:885618-31-5
CAS#:885618-31-5