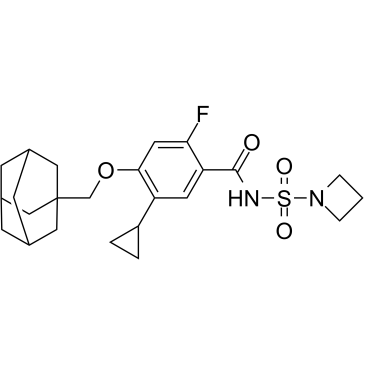
1494581-70-2
| Name | GDC-0276 |
|---|
| Description | GDC-0276 is a potent, selective, reversible and orally active NaV1.7 inhibitor with an IC50 value of 0.4 nM. GDC-0276 is well tolerated and exhibits a good pharmacokinetic profile. GDC-0276 has the potential for the treatment of pain and to address shortcomings of existing pain medications, such as addiction and off-target side effects[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50:0.4 nM (NaV1.7 Electrophysiology)[1] |
| In Vivo | GDC-0276 (oral adminstration; 0.5-5 mg/kg) shows enrichment of 14C with observed specific activities of 22.6 µCi/mg. GDC-0276 is not detected in urine; however, metabolites in urine were enriched in 14C with observed specific activities of 19.6 µCi/mg[3]. Animal Model: Six drug-naïve beagle dogs Group 1 four dogs (n=2 per sex) and Group 2 2 BDC dogs (n=2 male)[3] Dosage: 0.5, 1, 2, 3, 4, 5 mg/kg Administration: Oral adminstration Result: Showed mean specific activities of 12.2 µCi/mg (a 24% enrichment) (n=4 animals) and 23.5 µCi/mg (a 139% enrichment) (n=2 animals) for Groups 1 and 2, respectively. |
| References |
| Molecular Formula | C24H31FN2O4S |
|---|---|
| Molecular Weight | 462.58 |