3-Chloropropiophenone
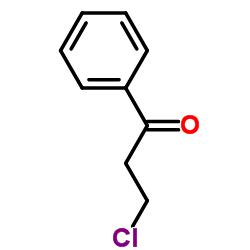
3-Chloropropiophenone structure
|
Common Name | 3-Chloropropiophenone | ||
|---|---|---|---|---|
| CAS Number | 936-59-4 | Molecular Weight | 168.62 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 263.3±23.0 °C at 760 mmHg | |
| Molecular Formula | C9H9ClO | Melting Point | 48-50 °C(lit.) | |
| MSDS | USA | Flash Point | 127.0±13.7 °C | |
Use of 3-Chloropropiophenone3-Chloropropiophenone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 3-Chloropropiophenone |
|---|---|
| Synonym | More Synonyms |
| Description | 3-Chloropropiophenone is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 3-氯苯丙酮是一种小分子化合物。 |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 263.3±23.0 °C at 760 mmHg |
| Melting Point | 48-50 °C(lit.) |
| Molecular Formula | C9H9ClO |
| Molecular Weight | 168.62 |
| Flash Point | 127.0±13.7 °C |
| Exact Mass | 168.034195 |
| PSA | 17.07000 |
| LogP | 2.23 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.527 |
| Stability | Stable. Incompatible with strong bases, strong oxidizing agents. |
| Water Solubility | Insoluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914700090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Synthesis of enantiopure glycidol derivatives via a one-pot two-step enzymatic cascade.
Org. Biomol. Chem. 13(7) , 2146-52, (2015) Styrene monooxygenase (SMO) can catalyze the kinetic resolution of secondary allylic alcohols to provide enantiopure glycidol derivatives. To overcome the low theoretical yield of kinetic resolution, ... |
|
|
Asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone by preheated immobilized Candida utilis.
Biotechnol. Lett. 31(12) , 1879-83, (2009) An efficient method for asymmetric reduction of (S)-3-chloro-1-phenylpropanol from 3-chloropropiophenone was developed using preheated Candida utilis cells immobilized in calcium alginate gel beads. H... |
|
|
Asymmetric Synthesis of (R)-Fluoxetine: A Practical Approach Using Recyclable and in-situ Generated Oxazaborolidine Catalyst. Padiya K, et al.
Chin. J. Chem. 27(6) , 1137-40, (2009)
|
| B-CHLOROPROPIOPHENONE |
| ω-Chloropropiophenone |
| 3-Chloro-1-phenylpropan-1-one |
| 3-chloro-1-phenyl-propan-1-one |
| Beta-Chloropropiophenone |
| 3-Chloropropanophenone |
| β-Chloropropiophenone |
| EINECS 213-317-6 |
| β-Chloroethyl phenyl ketone |
| 3-Chloropropiophenone |
| 3-Chloro-1-phenyl-1-propanone |
| 2-Chloro-1-benzoylethane |
| Propiophenone, 3-chloro- |
| Propiophenone,3-chloro |
| PhCOCH2CH2Cl |
| 1-Propanone, 3-chloro-1-phenyl- |
| MFCD00000990 |
 CAS#:625-36-5
CAS#:625-36-5 CAS#:71-43-2
CAS#:71-43-2 CAS#:104-52-9
CAS#:104-52-9 CAS#:768-03-6
CAS#:768-03-6 CAS#:93-55-0
CAS#:93-55-0 CAS#:18776-12-0
CAS#:18776-12-0 CAS#:98-88-4
CAS#:98-88-4 CAS#:2674-04-6
CAS#:2674-04-6 CAS#:50-00-0
CAS#:50-00-0 CAS#:98-86-2
CAS#:98-86-2 CAS#:3375-38-0
CAS#:3375-38-0![cis-1,5-diphenyl-2,6-dioxa[3.3.0]bicyclooctane structure](https://image.chemsrc.com/caspic/295/127824-37-7.png) CAS#:127824-37-7
CAS#:127824-37-7 CAS#:3506-36-3
CAS#:3506-36-3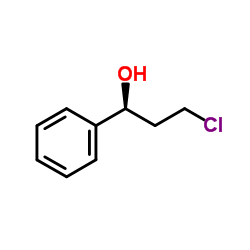 CAS#:100306-34-1
CAS#:100306-34-1 CAS#:613-87-6
CAS#:613-87-6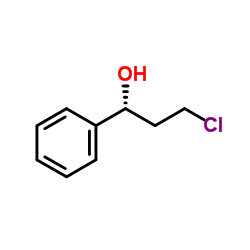 CAS#:100306-33-0
CAS#:100306-33-0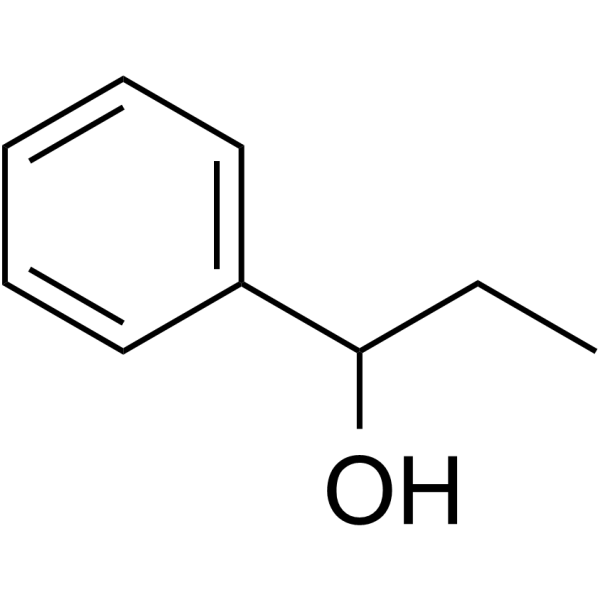 CAS#:93-54-9
CAS#:93-54-9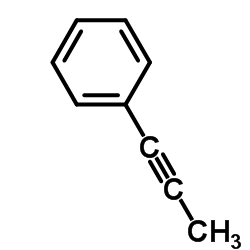 CAS#:673-32-5
CAS#:673-32-5
