3-Chloropropanoyl chloride
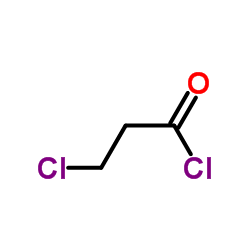
3-Chloropropanoyl chloride structure
|
Common Name | 3-Chloropropanoyl chloride | ||
|---|---|---|---|---|
| CAS Number | 625-36-5 | Molecular Weight | 126.969 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 144.9±13.0 °C at 760 mmHg | |
| Molecular Formula | C3H4Cl2O | Melting Point | -32 °C | |
| MSDS | Chinese USA | Flash Point | 61.7±0.0 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
| Name | 3-Chloropropionyl chloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 144.9±13.0 °C at 760 mmHg |
| Melting Point | -32 °C |
| Molecular Formula | C3H4Cl2O |
| Molecular Weight | 126.969 |
| Flash Point | 61.7±0.0 °C |
| Exact Mass | 125.963921 |
| PSA | 17.07000 |
| LogP | 1.06 |
| Vapour Pressure | 5.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.437 |
| Storage condition | Flammables area |
| Water Solubility | reacts |
Synonym:None Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: 10 14 26 35 Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Flammable. Reacts violently with water. Very toxic by inhalation. Causes severe burns.Corrosive.Lachrymator (substance which increases the flow of tears). Potential Health Effects Eye: Causes eye burns. Lachrymator (substance which increases the flow of tears). Skin: Causes severe burns with delayed tissue destruction. Ingestion: May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. Inhalation: Causes chemical burns to the respiratory tract. Inhalation may be fatal as a result of spasm, inflammation, edema of the larynx and bronchi, chemical pneumonitis and pulmonary edema. Vapors may cause dizziness or suffocation. Chronic: None Section 4 - FIRST AID MEASURES Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes). Skin: Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes. Ingestion: Do not induce vomiting. If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid immediately. Inhalation: Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. Notes to Physician: Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air. Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Water Reactive. Material will react with water and may release a flammable and/or toxic gas. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. May ignite or explode on contact with steam or moist air. Extinguishing Media: Use dry sand or earth to smother fire. Do NOT use water directly on fire. Use water spray to cool fire-exposed containers. Use foam, dry chemical, or carbon dioxide. Do NOT use straight streams of water. Contact professional fire-fighters immediately. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. Do not expose spill to water. A vapor suppressing foam may be used to reduce vapors. Section 7 - HANDLING and STORAGE Handling: Do not allow water to get into the container because of violent reaction. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame. Use with adequate ventilation. Use only in a chemical fume hood. Discard contaminated shoes. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames. Storage: Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a cool, dry place. Keep container closed when not in use. Store in a tightly closed container. Keep away from water. Flammables-area. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use explosion-proof ventilation equipment. Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 625-36-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to minimize contact with skin. Respirators: A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Liquid Color: clear, colorless Odor: Stench pH: Not available. Vapor Pressure: 10 mbar @ 20 deg C Viscosity: 1.4 mPas 20 deg C Boiling Point: 143 - 145 deg C @ 760.00mm Hg Freezing/Melting Point: -32 deg C Autoignition Temperature: 490 deg C ( 914.00 deg F) Flash Point: 30 deg C ( 86.00 deg F) Explosion Limits, lower: 8.80 vol % Explosion Limits, upper: 20.20 vol % Decomposition Temperature: 30 deg C Solubility in water: Specific Gravity/Density: 1.3300g/cm3 Molecular Formula: C3H4Cl2O Molecular Weight: 126.97 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Combines vigorously or explosively with water. Conditions to Avoid: Incompatible materials, ignition sources, excess heat, exposure to moist air or water. Incompatibilities with Other Materials: Acids, oxidizing materials, water. Hazardous Decomposition Products: Hydrogen chloride, phosgene, carbon monoxide, carbon dioxide. Hazardous Polymerization: Has not been reported. Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 625-36-5 unlisted. LD50/LC50: Not available. Not available. Carcinogenicity: 3-CHLOROPROPIONYL CHLORIDE - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S.* Hazard Class: 6.1 (8) UN Number: 2927 Packing Group: II IMO Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S. Hazard Class: 6.1 (8) UN Number: 2927 Packing Group: II RID/ADR Shipping Name: TOXIC LIQUID, CORROSIVE, ORGANIC, N.O.S. Hazard Class: 6.1 UN Number: 2927 Packing group: II Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: T+ C Risk Phrases: R 10 Flammable. R 14 Reacts violently with water. R 26 Very toxic by inhalation. R 35 Causes severe burns. Safety Phrases: S 8 Keep container dry. S 9 Keep container in a well-ventilated place. S 16 Keep away from sources of ignition - No smoking. S 33 Take precautionary measures against static discharges. S 36/37/39 Wear suitable protective clothing, gloves and eye/face protection. S 38 In case of insufficient ventilation, wear suitable respiratory equipment. S 45 In case of accident or if you feel unwell, seek medical advice immediately (show the label where possible). WGK (Water Danger/Protection) CAS# 625-36-5: 1 Canada CAS# 625-36-5 is listed on Canada's NDSL List. CAS# 625-36-5 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 625-36-5 is listed on the TSCA inventory. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H314-H330 |
| Supplemental HS | Reacts violently with water. |
| Precautionary Statements | P260-P280-P284-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R10;R14;R22;R26;R35 |
| Safety Phrases | S23-S26-S36/37/39-S45-S38-S16-S8 |
| RIDADR | UN 3390 6.1/PG 1 |
| WGK Germany | 1 |
| Packaging Group | I |
| Hazard Class | 6.1 |
| HS Code | 29159080 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2915900090 |
|---|---|
| Summary | 2915900090 other saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:5.5% General tariff:30.0% |
|
Macroporous and hydrophilic polymer resins modified with isothiocyanate groups for immobilization of enzymes.
Biotechnol. Bioeng. 36(3) , 219-23, (1990) Hydrophilic and macroporous polymer resins composed of glycerylmethacrylate, styrene, and divinylbenzene were quite easily modified with isothiocyanate groups using a Friedel-Crafts reaction with 3-ch... |
|
|
Diethylaminopropionamido-hydroxy-anthraquinones as potential anticancer agents: synthesis and characterization.
Arch. Pharm. (Weinheim) 322(9) , 541-4, (1989) A number of new 9,10-anthracenediones were obtained, bearing one or two hydroxyl groups and one positively charged side chain at different positions of the aromatic ring system (compounds 1-5 in Chart... |
|
|
Microwave-enhanced liquid-phase synthesis of thiohydantoins and thioxotetrahydropyrimidinones.
Mol. Divers. 7(2-4) , 185-98, (2003) An efficient, microwave-assisted method for the liquid-phase combinatorial synthesis of 3,5-disubstituted thiohydantoins and 3,5-disubstituted 2-thioxotetrahydropyrimidin-4-ones has been developed. In... |
| MFCD00000747 |
| 3-Chloropropinyl chloride |
| b-Chloropropanoyl chloride |
| Chloropropionyl chloride |
| 3-chloro-propanoylchlorid |
| 3-chloro propionic acid chloride |
| 3-chloro-n-propionyl chloride |
| β-Chloropropionoyl chloride |
| β-Chloropropanoyl chloride |
| EINECS 210-890-4 |
| 3-Chloropropionyl chloride |
| 3-Chloropropionyl ch |
| β-Chloropropionyl chloride |
| 3-Chlorpropionylchlorid |
| 3-Chloropropanoyl chloride |
| 3-Chloropropionyl chlorode |
| Propanoyl chloride, 3-chloro- |
| Propionyl chloride, 3-chloro- |
 CAS#:79-10-7
CAS#:79-10-7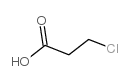 CAS#:107-94-8
CAS#:107-94-8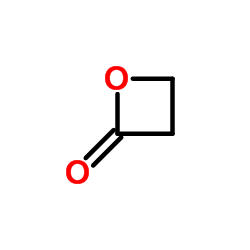 CAS#:57-57-8
CAS#:57-57-8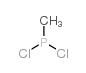 CAS#:676-83-5
CAS#:676-83-5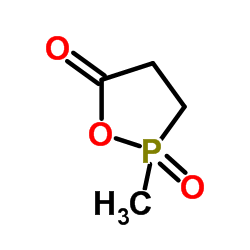 CAS#:15171-48-9
CAS#:15171-48-9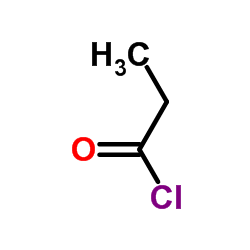 CAS#:79-03-8
CAS#:79-03-8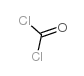 CAS#:75-44-5
CAS#:75-44-5 CAS#:54509-73-8
CAS#:54509-73-8 CAS#:201230-82-2
CAS#:201230-82-2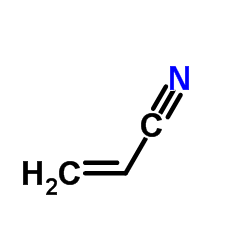 CAS#:107-13-1
CAS#:107-13-1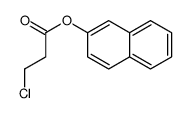 CAS#:111709-01-4
CAS#:111709-01-4 CAS#:111550-38-0
CAS#:111550-38-0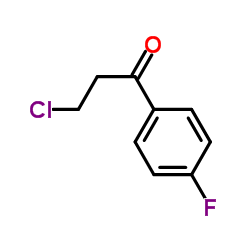 CAS#:347-93-3
CAS#:347-93-3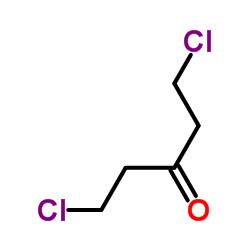 CAS#:3592-25-4
CAS#:3592-25-4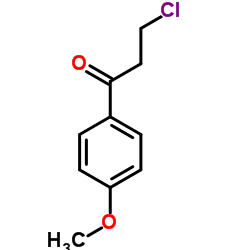 CAS#:35999-20-3
CAS#:35999-20-3 CAS#:4600-86-6
CAS#:4600-86-6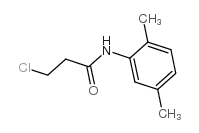 CAS#:39494-07-0
CAS#:39494-07-0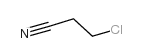 CAS#:542-76-7
CAS#:542-76-7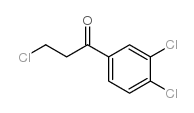 CAS#:35857-66-0
CAS#:35857-66-0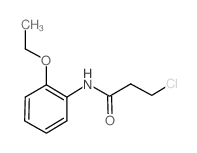 CAS#:334504-88-0
CAS#:334504-88-0
