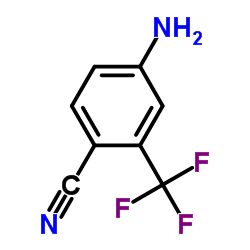
Enzalutamide (MDV3100)

Enzalutamide (MDV3100) structure
|
Common Name | Enzalutamide (MDV3100) | ||
|---|---|---|---|---|
| CAS Number | 915087-33-1 | Molecular Weight | 464.436 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C21H16F4N4O2S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Enzalutamide (MDV3100)Enzalutamide (MDV3100) is an androgen receptor (AR) antagonist with an IC50 of 36 nM in LNCaP prostate cells. |
| Name | enzalutamide |
|---|---|
| Synonym | More Synonyms |
| Description | Enzalutamide (MDV3100) is an androgen receptor (AR) antagonist with an IC50 of 36 nM in LNCaP prostate cells. |
|---|---|
| Related Catalog | |
| Target |
IC50: 36 nM (androgen-receptor, in LNCaP cells)[1] |
| In Vitro | Enzalutamide has greater affinity to AR than Bicalutamide does in a competition assay with 16β-[18F]fluoro-5α-DHT (18-FDHT) in castration-resistant LNCaP/AR cells (AR-overexpressing). While Enzalutamide shows no agonism in LNCaP/AR prostate cells. Enzalutamide antagonizes induction of prostate-specific antigen (PSA) and transmembrane serine protease 2 (TMPRSS2), combination with the synthetic androgen R1881 in parental LNCaP cells. Enzalutamide inhibits the transcriptional activity of a mutant AR protein (W741C, mutation of Trp741 to Cys)[1]. Enzalutamide also prevents nuclear translocation and co-activator recruitment of the ligand-receptor complex[2]. |
| In Vivo | Enzalutamide induces great tumor regression in castrate male mice bearing LNCaP/AR xenografts at a dose of 10 mg/kg[1]. Enzalutamide shows dose-independent pharmacokinetics at intravenous and oral doses of 0.5-5 mg/kg[4]. |
| Cell Assay | LNCaP cells (107 cells/condition) are grown in RPMI media supplemented with 5% charcoalstripped serum for 22 days, then treated with DMSO or 1 nM R1881, combined with an antiandrogen (DMSO, 1 μM Bicalutamide, 10 μM Bicalutamide, 1 μM RD162, 10 μM RD162, 1 μM MDV3100, or 10 μM MDV3100) for 8 hours. An aliquot of cells are harvested for qRT-PCR of PSA and TMPRSS2 mRNA. The remaining cells are cross-linked using 1% paraformaldehyde for 10 minutes, then glycine is added and samples centrifuged (4°C, 4000 rpm, 5 minutes) to stop further crosslinking. Chromatin immunoprecipitation is performed using a chromatin immunoprecipitation assay kit. Immunoprecipitated DNA is amplified by real-time PCR. Primers are PSA enhancer forward-ATGTTCACATTAGTACACCTTGCC and reverse-TCTCAGATCCAGGCTTGCTTACTGTC and TMPRSS2 enhancer forward-TGGTCCTGGATGATAAAAAAAGTTT and reverse-GACATACGCCCCACAACAGA[1]. |
| Animal Admin | Mice[3] Following a 5-day acclimation period, 5- to 9-week-old male CB17SCID mice are castrated and allowed to recover for an additional 5 days before inoculation with tumor cells. LNCaP cells co-expressing exogenous AR and the AR-dependent reporter construct ARR2-Pb-Luc (LNCaP-AR-Lux cells) are used to generate a xenograft model of human prostate cancer. Before implantation, LNCaP-AR-Lux cells are prepared by the addition of trypsin-EDTA, washed with complete medium, collected and resuspended at 20×106 cells/mL. Cell suspensions are diluted with Matrigel to 2×106 cells/0.2 mL and delivered subcutaneously in the suprascapular region. Tumor growth is monitored to the volume of 100 mm3 when treatment begins (80 days). The observed rate of tumor take with LNCaP-AR-Lux cells is between 70% and 80%. Body weight and tumor volumes (width2×length/2) are measured two to three times per week with a digital caliper, and the average tumor volumes are determined. Test drugs are diluted in Tween 80:PEG 400, and stored at 4°C until administration by oral gavage. Each group of mice (n=7) is treated daily for 28 consecutive days with 1, 10, or 50 mg/kg Enzalutamide, vehicle control, or 50 mg/kg Bicalutamide. At the end of the treatment period or when tumor volume exceeded 1,000 mm3, animals are euthanized and blood and tissue samples are collected for analysis. Rats[4] Male SD rats (n=3) are administered Enzalutamide through the tail vein (intravenous) and by oral gavage at 1 mg/kg and are kept in metabolic cages after dosing. Urine and feces samples are collected over the following time intervals after dosing: 0-2, 2-4, 4-6, 6-10, 10-24, 24-48, and 48-72 h. The metabolic cages are rinsed with distilled water, and residues are added to the urine samples at 72 h. To extract the Enzalutamide present in the feces, samples are shaken vigorously for 12 h with 50 % methanol. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C21H16F4N4O2S |
| Molecular Weight | 464.436 |
| Exact Mass | 464.093018 |
| PSA | 108.53000 |
| LogP | 2.13 |
| Index of Refraction | 1.630 |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933990090 |
|
~25% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: Regents of the University of California Patent: US2007/254933 A1, 2007 ; Location in patent: Page/Page column 7 ; |
|
~79% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: MEDIVATION PROSTATE THERAPEUTICS, INC.; JAIN, Rajendra, Parasmal; ANGELAUD, Remy; THOMPSON, Andrew; LAMBERSON, Carol; GREENFIELD, Scott Patent: WO2011/106570 A1, 2011 ; Location in patent: Page/Page column 46 ; |
|
~4% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: MEDIVATION PROSTATE THERAPEUTICS, INC.; JAIN, Rajendra, Parasmal; ANGELAUD, Remy; THOMPSON, Andrew; LAMBERSON, Carol; GREENFIELD, Scott Patent: WO2011/106570 A1, 2011 ; Location in patent: Page/Page column 48 ; |
|
~% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: WO2011/106570 A1, ; |
|
~% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: WO2011/106570 A1, ; |
|
~% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: WO2011/106570 A1, ; |
|
~% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: WO2011/106570 A1, ; |
|
~% 
Enzalutamide (M... CAS#:915087-33-1 |
| Literature: WO2011/106570 A1, ; |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| MDV3100 |
| MDV 3100 |
| Enzalutamide |
| 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-sulfanylideneimidazolidin-1-yl]-2-fluoro-N-methylbenzamide |
| Enzalutamidum |
| 4-(3-(4-Cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-me |
| Xtandi |
| 4-{3-[4-Cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl}-2-fluoro-N-methylbenzamide |
| UNII-93T0T9GKNU |
| Benzamide, 4-[3-[4-cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methyl- |
| Enzalutamida |
| [14C]-Enzalutamide |
| MDV-3100 |
| 4-(3-(4-Cyano-3-(trifluoromethyl)phenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl)-2-fluoro-N-methylbenzamide |
| N-methyl-4-[3-(4-cyano-3-trifluoromethylphenyl)-5,5-dimethyl-4-oxo-2-thioxoimidazolidin-1-yl]-2-fluorobenzamide |
| 4-[3-[4-Cyano-3-(trifluoromethyl)phenyl]-5,5-dimethyl-4-oxo-2-thioxo-1-imidazolidinyl]-2-fluoro-N-methylbenzamide |
![4-[(2-Cyanopropan-2-yl)amino]-2-fluoro-N-methylbenzamide structure](https://image.chemsrc.com/caspic/085/915087-32-0.png)

![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine methyl ester structure](https://image.chemsrc.com/caspic/377/1332524-01-2.png)
![4-[1-(4-cyano-3-trifluoromethyl-phenylcarbamoyl)-1-methyl-ethylamino]-2-fluoro-N- structure](https://image.chemsrc.com/caspic/470/1289942-55-7.png)

![N-[3-Fluoro-4-[(methylamino)carbonyl]phenyl]-2-methylalanine structure](https://image.chemsrc.com/caspic/215/1289942-66-0.png)