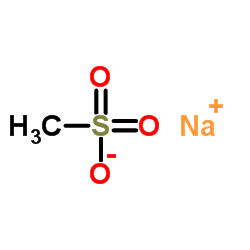
Nafamostat mesylate
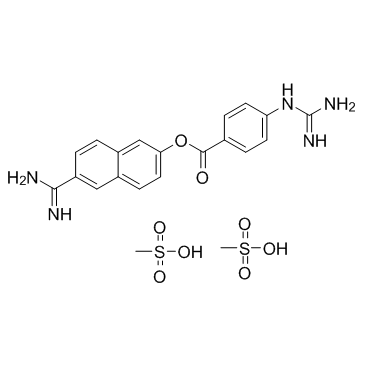
Nafamostat mesylate structure
|
Common Name | Nafamostat mesylate | ||
|---|---|---|---|---|
| CAS Number | 82956-11-4 | Molecular Weight | 539.58 | |
| Density | N/A | Boiling Point | 637.2ºCat 760 mmHg | |
| Molecular Formula | C21H25N5O8S2 | Melting Point | 259-261°C | |
| MSDS | Chinese USA | Flash Point | 339.1ºC | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of Nafamostat mesylateNafamostat mesylate, a synthetic serine protease inhibitor, is an anticoagulant.Target: Serine ProteaseTranilast (FUT-175) is an antiallergic drug for bronchial asthma. It has been used for the treatment of allergic disorders such as asthma, allergic rhinitis and atopic dermatitis. It has also been investigated for use as an antiproliferative drug on drug-eluting stents.A 20-40 mg/h dose of FUT-175 prolonged coagulation time sufficiently in the instrumental blood of the extracorporeal circuit but not in the systemic blood. Its anticoagulant activity decreased immediately after hemodialysis. Therefore, we could manage all patients without any bleeding trouble during hemodialysis with FUT-175 as an anticoagulant. Although there were side effects of FUT-175, such as nausea, vomiting, itching and eruption, they were not serious, and FUT-175 could be administered without interruption. FUT-175 seems to be useful as an anticoagulant during hemodialysis for patients susceptible to bleeding. |
| Name | Nafamostat Mesylate |
|---|---|
| Synonym | More Synonyms |
| Description | Nafamostat mesylate, a synthetic serine protease inhibitor, is an anticoagulant.Target: Serine ProteaseTranilast (FUT-175) is an antiallergic drug for bronchial asthma. It has been used for the treatment of allergic disorders such as asthma, allergic rhinitis and atopic dermatitis. It has also been investigated for use as an antiproliferative drug on drug-eluting stents.A 20-40 mg/h dose of FUT-175 prolonged coagulation time sufficiently in the instrumental blood of the extracorporeal circuit but not in the systemic blood. Its anticoagulant activity decreased immediately after hemodialysis. Therefore, we could manage all patients without any bleeding trouble during hemodialysis with FUT-175 as an anticoagulant. Although there were side effects of FUT-175, such as nausea, vomiting, itching and eruption, they were not serious, and FUT-175 could be administered without interruption. FUT-175 seems to be useful as an anticoagulant during hemodialysis for patients susceptible to bleeding. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 637.2ºCat 760 mmHg |
|---|---|
| Melting Point | 259-261°C |
| Molecular Formula | C21H25N5O8S2 |
| Molecular Weight | 539.58 |
| Flash Point | 339.1ºC |
| PSA | 200.82000 |
| LogP | 4.90630 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~41% 
Nafamostat mesylate CAS#:82956-11-4 |
| Literature: Aoyama; Okutome; NakayamaT.; Yaegashi; Matsui; Nunomura; Kurumi; Sakurai; Fujii Chemical and Pharmaceutical Bulletin, 1985 , vol. 33, # 4 p. 1458 - 1471 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
A novel protocol allowing oral delivery of a protein complement inhibitor that subsequently targets to inflamed colon mucosa and ameliorates murine colitis.
Clin. Exp. Immunol. 177(2) , 500-8, (2014) While there is evidence of a pathogenic role for complement in inflammatory bowel disease, there is also evidence for a protective role that relates to host defence and protection from endotoxaemia. T... |
|
|
Elevation of the terminal complement activation products C5a and C5b-9 in ALS patient blood.
J. Neuroimmunol. 276(1-2) , 213-8, (2015) Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease, characterized by the progressive loss of motor neurons within the central nervous system. Neural degeneration and inflammatory... |
|
|
TMPRSS2, a novel membrane-anchored mediator in cancer pain.
Pain 156 , 923-30, (2015) More than half of all cancer patients have significant pain during the course of their disease. The strategic localization of TMPRSS2, a membrane-bound serine protease, on the cancer cell surface may ... |
| Benzoic acid, 4-[(diaminomethylene)amino]-, 6-[(Z)-aminoiminomethyl]-2-naphthalenyl ester, methanesulfonate (1:1) |
| NAFAMOSTAT, MESYLATE |
| Nafamostatmesylate |
| MFCD00941430 |
| Nafamostat mesilate |
| 6-Amidino-2-naphthyl 4-guanidino-benzoate dimethanesulphonate |
| Nafamostat . mesylate |
| 6-carbamimidoylnaphthalen-2-yl 4-carbamimidamidobenzoate methanesulfonate (1:1) |
| NAFAMOSTAT MESYLATE |
| mostat mesylate |
| 6-Carbamimidoyl-2-naphthyl 4-[(diaminomethylene)amino]benzoate methanesulfonate (1:1) |
| 6-[amino(imino)methyl]-2-naphthyl 4-{[amino(imino)methyl]amino}benzoate dimethanesulfonate |
| Nafamostat (mesylate) |
| NAFAMOSTATMESILATE |