Atractylenolide I
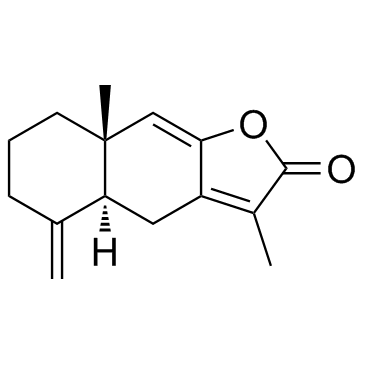
Atractylenolide I structure
|
Common Name | Atractylenolide I | ||
|---|---|---|---|---|
| CAS Number | 73069-13-3 | Molecular Weight | 230.302 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 405.0±44.0 °C at 760 mmHg | |
| Molecular Formula | C15H18O2 | Melting Point | 121-123 °C | |
| MSDS | USA | Flash Point | 170.8±25.9 °C | |
Use of Atractylenolide IAtractylenolide I is a sesquiterpene derived from the rhizome of Atractylodes macrocephala, possesses diverse bioactivities, such as neuroprotective, anti-allergic, anti-inflammatory and anticancer properties. Atractylenolide I reduces protein levels of phosphorylated JAK2 and STAT3 in A375 cells, and acts as a TLR4-antagonizing agent. |
| Name | Atractylenolide I |
|---|---|
| Synonym | More Synonyms |
| Description | Atractylenolide I is a sesquiterpene derived from the rhizome of Atractylodes macrocephala, possesses diverse bioactivities, such as neuroprotective, anti-allergic, anti-inflammatory and anticancer properties. Atractylenolide I reduces protein levels of phosphorylated JAK2 and STAT3 in A375 cells, and acts as a TLR4-antagonizing agent. |
|---|---|
| Related Catalog | |
| Target |
JAK2, STAT3[1], TLR4[4] |
| In Vitro | Atractylenolide I (40, 60, 80, 100, 120, 150 μM) dose- and time-dependently reduces the cell viability in human A375 melanoma cells after treatment for 24, 48 and 72 hours. Atractylenolide I (50 and 100 μM) induces apoptosis of A375 cells in a dose-dependent manner at 48 h of treatment. Atractylenolide I (100 μM) significantly reduces protein levels of phosphorylated JAK2 and STAT3 in A375 cells, without effect on total JAK2 and STAT3. Furthermore, Atractylenolide I inhibits the mRNA expression of STAT3-targeted genes, including Bcl-xL, MMP-2 and MMP-9[1]. Atractylenolide I (up to 100 μM) shows no toxicity in normal cells. Atractylenolide I (25, 50 μM) decreases the Ox-LDL induced TNF-α, IL-6 and NO production in VSMCs. Atractylenolide I (12.5, 25 or 50 μM) significantly reduces the level of MCP-1 and inhibits Ox-LDL-induced VSMCs proliferation and migration. Atractylenolide I (25, 50 μM) inhibits positive staining of foam cells, and also significantly decreases lipid accumulation. Atractylenolide I (50 μM) suppresses p38MAPK and NF-κB p65 expression in VSMCs stimulated by Ox-LDL[3]. Atractylenolide I (1, 10, 100 μM) downregulates paclitaxel-induced expression of VEGF and survivin via MyD88-dependent TLR4 signaling in EOC cells[4]. |
| In Vivo | Atractylenolide I (5, 10 or 20 mg/kg, p.o.) restores the decreased body weight in mice subjected to chronic unpredictable mild stress (CUMS). Atractylenolide I alleviates CUMS-induced depressive-like behavior, attenuates CUMS-induced imbalances in hippocampal neurotransmitter levels and reduces CUMS-induced increases in hippocampal pro-inflammatory cytokine levels and in the NLRP3 inflammasome in the hippocampi of mice[2]. |
| Cell Assay | Briefly, serum starved VSMCs are pre-treated with indicated concentration of Atractylenolide I for 1 h followed by stimulation with Ox-LDL for 24 h. The purple formazan crystals formed after addition of MTT are solubilized in DMSO and absorbance is measured at 540 nm. The viability or proliferation rate is calculated as percentage of control (untreated VSMCs)[3]. |
| Animal Admin | Mice[2] After adaption for one week, 48 male ICR mice are randomly divided into six groups (eight mice per group): Control group (unstressed + saline vehicle), model group (CUMS + saline vehicle), three Atractylenolide I treatment groups (CUMS + Atractylenolide I) and a fluoxetine group (CUMS + FLU). From the 4th week, Atractylenolide I (5, 10 or 20 mg/kg) or fluoxetine (20 mg/kg) is daily administered by oral gavage for 3 weeks. After the last administration of Atractylenolide I or fluoxetine, behavioral tests are performed[2]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 405.0±44.0 °C at 760 mmHg |
| Melting Point | 121-123 °C |
| Molecular Formula | C15H18O2 |
| Molecular Weight | 230.302 |
| Flash Point | 170.8±25.9 °C |
| Exact Mass | 230.130676 |
| PSA | 26.30000 |
| LogP | 3.77 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.555 |
| Storage condition | 2-8°C |
| Water Solubility | methanol: soluble1mg/mL, clear, colorless |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
|
GABA(A) receptor modulators from Chinese herbal medicines traditionally applied against insomnia and anxiety.
Phytomedicine 19 , 334-340, (2012) Several Chinese herbal medicines (CHMs) are used in the treatment of insomnia, restlessness, or anxiety. However, mechanisms underlying this effect and scientific proof for their traditional use is sc... |
|
|
Quantitative analysis of atractylenolide I in rat plasma by LC–MS/MS method and its application to pharmacokinetic study
J. Pharm. Biomed. Anal. 58 , 172-176, (2012) Highlights ► We develop an HPLC/MS method for quantification of atractylenolide I in rat plasma. ► Pharmacokinetics was performed in rats ingested with 20 g/kg Atractylodis extract. ► Atractylenolide ... |
|
|
Screening for compounds with aromatase inhibiting activities from Atractylodes macrocephala Koidz.
Molecules 16 , 3146-3151, (2011) Ten compounds were isolated from the dichloromethane extract of Atractylodes macrocephala and their aromatase inhibiting activities were tested using an in vitro fluorescent-based aromatase assay. The... |
| Naphtho[2,3-b]furan-2(4H)-one, 4a,5,6,7,8,8a-hexahydro-3,8a-dimethyl-5-methylene-, (4aS,8aS)- |
| (4aS,8aS)-3,8a-Dimethyl-5-methylene-4a,5,6,7,8,8a-hexahydronaphtho[2,3-b]furan-2(4H)-one |
| Atractylenolide-1 |

