Melatonine
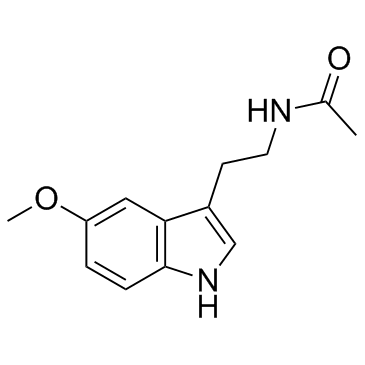
Melatonine structure
|
Common Name | Melatonine | ||
|---|---|---|---|---|
| CAS Number | 73-31-4 | Molecular Weight | 232.278 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 459.8±55.0 °C at 760 mmHg | |
| Molecular Formula | C13H16N2O2 | Melting Point | 116.5-118 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 231.9±31.5 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of MelatonineMelatonin is a hormone made by the pineal gland that can activates melatonin receptor. Melatonin plays a role in sleep and possesses important antioxidative and anti-inflammatory properties. |
| Name | melatonin |
|---|---|
| Synonym | More Synonyms |
| Description | Melatonin is a hormone made by the pineal gland that can activates melatonin receptor. Melatonin plays a role in sleep and possesses important antioxidative and anti-inflammatory properties. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vivo | Melatonin increases the levels of activated PTEN, RSK-1, mTOR and AMPKα kinases, mildly inhibits ERK-1/2 phosphorylation and Bad phosphorylation, significantly inhibits phosphorylations of S6 Ribosomal Protein, 4E-BP1, GSK-3α and GSK-3β, and slightly increases PRAS40 phosphorylation in animals[1]. Melatonin ameliorates the neurotoxiciy and astrocyte activation induced by Aβ1-42 in the cerebral cortex. Melatonin also blocks the reduction in Reelin and Dab1 expression induced by Aβ1-42[2]. Melatonin treatment and lack of NLRP3-/- share similar inhibition of NF-κB and NLRP3 signaling pathway in mice. Melatonin treatment and lack of NLRP3-/- share some patterns of clock genes expression, and improve cardiomyocytes morphology in mice[3]. |
| Animal Admin | A total of two sets of adult male C57BL/6j mice weighing 21-26 g are randomly assigned to one of four groups and treated with intraperitoneal (i.p.) delivery of (i) vehicle (50 μL isotonic saline/5% ethanol), (ii) melatonin (4 mg/kg, dissolved in 0.9% isotonic saline/5% ethanol), (iii) Wortmannin, and (iv) melatonin/Wortmannin immediately after reperfusion. In the first set, mice are exposed to 30 min of focal cerebral ischemia (FCI) and 72 h reperfusion for the evaluation of disseminate ischemic injury in the striatum, and signaling pathway analysis (n=7 per group). The second group of mice is exposed to 90 min of FCI and 24 h reperfusion for the analysis of infarct development, brain swelling and IgG extravasation (n=7 per group). |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 459.8±55.0 °C at 760 mmHg |
| Melting Point | 116.5-118 °C(lit.) |
| Molecular Formula | C13H16N2O2 |
| Molecular Weight | 232.278 |
| Flash Point | 231.9±31.5 °C |
| Exact Mass | 232.121185 |
| PSA | 54.12000 |
| LogP | 1.94 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.580 |
| InChIKey | DRLFMBDRBRZALE-UHFFFAOYSA-N |
| SMILES | COc1ccc2[nH]cc(CCNC(C)=O)c2c1 |
| Storage condition | -15°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | T |
| Risk Phrases | R60 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AC5955000 |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Cranberry flavonoids prevent toxic rat liver mitochondrial damage in vivo and scavenge free radicals in vitro.
Cell Biochem. Funct. 33 , 202-10, (2015) The present study was undertaken for further elucidation of the mechanisms of flavonoid biological activity, focusing on the antioxidative and protective effects of cranberry flavonoids in free radica... |
|
|
Oxidative damage of rat liver mitochondria during exposure to t-butyl hydroperoxide. Role of Ca²⁺ ions in oxidative processes.
Life Sci. 92(23) , 1110-7, (2013) The present study was designed for further evaluation of the biochemical mechanism of hepatic mitochondrial dysfunction under oxidative damages induced by organic hydroperoxide, tert-butyl hydroperoxi... |
|
|
Development of Man-rGO for Targeted Eradication of Macrophage Ablation.
Mol. Pharm. 12 , 3226-36, (2015) This study was aimed to develop and evaluate a smart nanosystem that targeted photothermal ablation of inflammatory macrophages in atherosclerotic plaque. Mannosylated-reduced graphene oxide (Man-rGO)... |
| Melatonin |
| EINECS 200-797-7 |
| N-(2-(5-methoxyindol-3-yl)ethyl)-Acetamide |
| Melovine |
| Melatonine |
| primex |
| Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]- |
| MLT |
| N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide |
| N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-Acetamide |
| N-[2-(5-methoxyindol-3-yl)ethyl]-Acetamide |
| N-acetyl-5-methoxytryptamine |
| Circadin |
| N-[2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide |
| MFCD00005655 |
 CAS#:608-07-1
CAS#:608-07-1 CAS#:108-24-7
CAS#:108-24-7 CAS#:1210-83-9
CAS#:1210-83-9 CAS#:77-78-1
CAS#:77-78-1 CAS#:692-33-1
CAS#:692-33-1 CAS#:201230-82-2
CAS#:201230-82-2 CAS#:19501-58-7
CAS#:19501-58-7 CAS#:28772-49-8
CAS#:28772-49-8 CAS#:2735-73-1
CAS#:2735-73-1![N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-3-oxobutanamide structure](https://image.chemsrc.com/caspic/329/112081-40-0.png) CAS#:112081-40-0
CAS#:112081-40-0 CAS#:50-67-9
CAS#:50-67-9 CAS#:362-43-6
CAS#:362-43-6![5-[3-[2-(acetylamino)ethyl]-5-methoxy-1H-indol-2-yl]-2',3'-O-(1-methylethylidene)uridine structure](https://image.chemsrc.com/caspic/121/68798-00-5.png) CAS#:68798-00-5
CAS#:68798-00-5 CAS#:3589-73-9
CAS#:3589-73-9![N-[2-(5-methoxy-4-nitro-1H-indol-3-yl)ethyl]acetamide structure](https://image.chemsrc.com/caspic/095/304854-99-7.png) CAS#:304854-99-7
CAS#:304854-99-7![6-methoxy-1-methyl-3,4-dihydro-2H-pyrido[3,4-b]indole,hydrochloride structure](https://image.chemsrc.com/caspic/258/2537-74-8.png) CAS#:2537-74-8
CAS#:2537-74-8![6-Acetyl-3-[2-(acetylamino)ethyl]-5-Methoxy-H-indole-1-carboxylic Acid Ethyl Ester structure](https://image.chemsrc.com/caspic/350/188397-05-9.png) CAS#:188397-05-9
CAS#:188397-05-9
