Dihydrochelerythrine
Modify Date: 2025-08-19 17:25:08

Dihydrochelerythrine structure
|
Common Name | Dihydrochelerythrine | ||
|---|---|---|---|---|
| CAS Number | 6880-91-7 | Molecular Weight | 349.380 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 565.9±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H19NO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 171.6±27.3 °C | |
Use of DihydrochelerythrineDihydrochelerythrine is a natural compound isolated from the leaves of Macleaya microcarpa; has antifungal activity.IC50 value:Target: in vitro: Dihydrochelerythrine showed the highest antifungal activity against B. cinerea Pers, with 98.32% mycelial growth inhibition at 50 μg/mL. Dihydrochelerythrine inhibited spore germination in vitro in a concentration-dependent manner [1]. Dihydrochelerythrine appeared to be less cytotoxic since the viability of cells exposed to 20 microM dihydrochelerythrine for 24h was reduced only to 53%. A dose-dependent induction of apoptosis and necrosis by chelerythrine and dihydrochelerythrine was confirmed by annexin V/propidium iodide dual staining flow cytometry [2]. Dihydrochelerythrine (4) exhibited strong activity against methicillin-resistant Staphylococcus aureus SK1 and moderate activity against Escherichia coli TISTR 780 with MIC values of 8 and 16 μg/mL, respectively [3]. |
| Name | 1,2-dimethoxy-12-methyl-13H-[1,3]benzodioxolo[5,6-c]phenanthridine |
|---|---|
| Synonym | More Synonyms |
| Description | Dihydrochelerythrine is a natural compound isolated from the leaves of Macleaya microcarpa; has antifungal activity.IC50 value:Target: in vitro: Dihydrochelerythrine showed the highest antifungal activity against B. cinerea Pers, with 98.32% mycelial growth inhibition at 50 μg/mL. Dihydrochelerythrine inhibited spore germination in vitro in a concentration-dependent manner [1]. Dihydrochelerythrine appeared to be less cytotoxic since the viability of cells exposed to 20 microM dihydrochelerythrine for 24h was reduced only to 53%. A dose-dependent induction of apoptosis and necrosis by chelerythrine and dihydrochelerythrine was confirmed by annexin V/propidium iodide dual staining flow cytometry [2]. Dihydrochelerythrine (4) exhibited strong activity against methicillin-resistant Staphylococcus aureus SK1 and moderate activity against Escherichia coli TISTR 780 with MIC values of 8 and 16 μg/mL, respectively [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 565.9±50.0 °C at 760 mmHg |
| Molecular Formula | C21H19NO4 |
| Molecular Weight | 349.380 |
| Flash Point | 171.6±27.3 °C |
| Exact Mass | 349.131409 |
| PSA | 40.16000 |
| LogP | 4.56 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.657 |
| Storage condition | 2-8℃ |
| HS Code | 2933990090 |
|---|
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| bocconoline |
| [1,3]Benzodioxolo[5,6-c]phenanthridine, 12,13-dihydro-1,2-dimethoxy-12-methyl- |
| Dihydrochelerythrine |
| (1,3)Benzodioxolo(5,6-c)phenanthridine, 12,13-dihydro-1,2-dimethoxy-12-methyl- |
| 5,6-dihydrochelerythrine |
| 12,13-dihydrochelerythrine |
| 1,2-Dimethoxy-12-methyl-12,13-dihydro[1,3]benzodioxolo[5,6-c]phenanthridine |
 CAS#:64-18-6
CAS#:64-18-6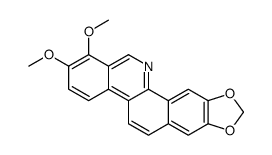 CAS#:6900-99-8
CAS#:6900-99-8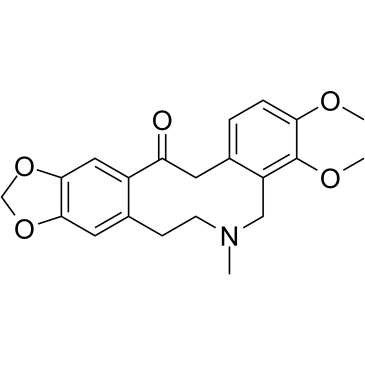 CAS#:485-91-6
CAS#:485-91-6 CAS#:34316-15-9
CAS#:34316-15-9 CAS#:77-78-1
CAS#:77-78-1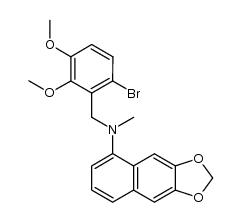 CAS#:610788-51-7
CAS#:610788-51-7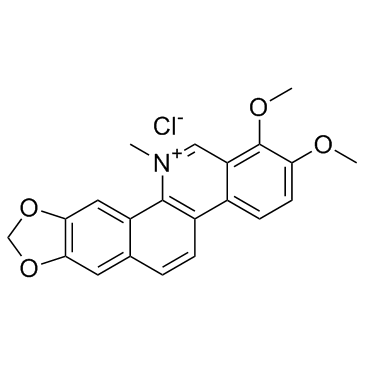 CAS#:3895-92-9
CAS#:3895-92-9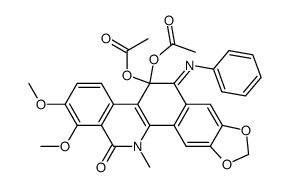 CAS#:72584-80-6
CAS#:72584-80-6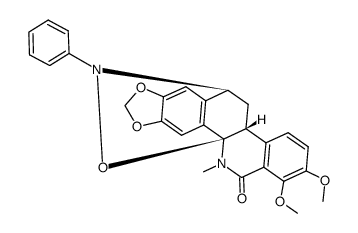 CAS#:67258-01-9
CAS#:67258-01-9 CAS#:28342-33-8
CAS#:28342-33-8
