7-Methoxy-1-tetralone
Modify Date: 2025-08-21 12:38:42
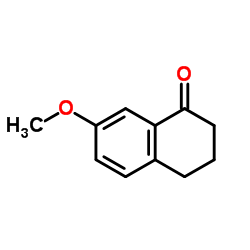
7-Methoxy-1-tetralone structure
|
Common Name | 7-Methoxy-1-tetralone | ||
|---|---|---|---|---|
| CAS Number | 6836-19-7 | Molecular Weight | 176.21 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 312.3±31.0 °C at 760 mmHg | |
| Molecular Formula | C11H12O2 | Melting Point | 59-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 145.8±18.4 °C | |
Use of 7-Methoxy-1-tetralone7-Methoxy-1-tetralone is a potent antitumor agent. 7-Methoxy-1-tetralone inhibits cancer cell proliferation and migration, and induces hepatocellular carcinoma cell (HCC) apoptosis. 7-Methoxy-1-tetralone decreased the protein levels of NF-κB, matrix metallopeptidase 2 (MMP2)/MMP9, and p-AKT. 7-Methoxy-1-tetralone showed antitumor activity in nude mice and had no effect on body weight and liver, spleen and organ index[1]. |
| Name | 7-Methoxy-1-tetralone |
|---|---|
| Synonym | More Synonyms |
| Description | 7-Methoxy-1-tetralone is a potent antitumor agent. 7-Methoxy-1-tetralone inhibits cancer cell proliferation and migration, and induces hepatocellular carcinoma cell (HCC) apoptosis. 7-Methoxy-1-tetralone decreased the protein levels of NF-κB, matrix metallopeptidase 2 (MMP2)/MMP9, and p-AKT. 7-Methoxy-1-tetralone showed antitumor activity in nude mice and had no effect on body weight and liver, spleen and organ index[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 7-Methoxy-1-tetralone (31.25-1000 μM; 48 h) 抑制 LO2 和 HepG2 细胞的增殖活性[1]。 7-Methoxy-1-tetralone (40 μM, 100 μM, 250 μM; 48 h) 诱导 HepG2 细胞凋亡,调节细胞中 c-Met、p-AKT、AKT、NF-κB、MMP2、MMP9 蛋白的表达水平[1]。 Western Blot Analysis[1] Cell Line: HepG2 cells Concentration: 40 μM, 100 μM, and 250 μM Incubation Time: 48 h Result: Decreased the protein expression levels of c-Met, p-AKT, NF-κB, MMP2, and MMP9. Cell Proliferation Assay[1] Cell Line: LO2 and HepG2 cells Concentration: 31.25 μM, 62.5 μM, 125 μM, 250 μM, 500 μM, and 1000 μM Incubation Time: 24 h, 48 h, and 72 h Result: Exhibited anti-proliferative activity of MT on LO2 and HepG2 cells. |
| In Vivo | 7-Methoxy-1-tetralone (80, 120, or 160 mg/kg/d;腹腔注射;共 19 次剂量) 抑制 HepG2 细胞皮下植入肿瘤模型的肿瘤生长[1]。 Animal Model: BALB/c nude mice (5-week-old) with subcutaneously implanted HepG2 cells[1] Dosage: 80, 120, or 160 mg/kg/d Administration: Intraperitoneal injection; sacrificed after 19 days Result: Resulted the tumor inhibition rates of 40.57% (80 mg/kg), 51.43% (120 mg/kg), 79.43% (160 mg/kg), respectively. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 312.3±31.0 °C at 760 mmHg |
| Melting Point | 59-63 °C(lit.) |
| Molecular Formula | C11H12O2 |
| Molecular Weight | 176.21 |
| Flash Point | 145.8±18.4 °C |
| Exact Mass | 176.083725 |
| PSA | 26.30000 |
| LogP | 2.79 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.549 |
| InChIKey | GABLTKRIYDNDIN-UHFFFAOYSA-N |
| SMILES | COc1ccc2c(c1)C(=O)CCC2 |
| Storage condition | 2~8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914509090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
The reaction of 1-tetralones with thallium trinitrate supported on clay: ring contraction vs alpha-oxidation. Ferraz H, et al.
J. Braz. Chem. Soc. 12(5) , 680-684, (2001)
|
|
|
Substituted Derivatives of 3a, 4, 5, 6-Tetrahydrosuccinimido [3, 4-b] Acenaphthen-10-one. Campaigne E and Weddleton RF.
Proc. Indian Acad. Sci. A 95 , (2013)
|
|
|
13H-benzo [6-7] indolo [3, 2-c] quinolines (B [6, 7] IQ): optimization of their DNA triplex-specific stabilization properties. Schmitt P, et al.
Chem. Commun. (Camb.) 9 , 763-764, (2000)
|
| EINECS 229-916-0 |
| 3,4-dihydro-7-methoxynaphthalen-1(2H)-one |
| 7-Methoxy-3,4-dihydronaphthalen-1(2H)-one |
| 1(2H)-Naphthalenone, 3,4-dihydro-7-methoxy- |
| MFCD00001696 |
| 7-Methoxy-3,4-dihydro-1(2H)-naphthalenone |
| 7-methoxy-3,4-dihydro-2H-naphthalen-1-one |
 CAS#:4521-28-2
CAS#:4521-28-2 CAS#:32820-10-3
CAS#:32820-10-3 CAS#:1730-48-9
CAS#:1730-48-9 CAS#:106336-27-0
CAS#:106336-27-0 CAS#:6836-18-6
CAS#:6836-18-6 CAS#:87543-05-3
CAS#:87543-05-3 CAS#:666177-27-1
CAS#:666177-27-1 CAS#:3153-44-4
CAS#:3153-44-4 CAS#:87543-11-1
CAS#:87543-11-1 CAS#:31846-36-3
CAS#:31846-36-3 CAS#:4133-34-0
CAS#:4133-34-0![8-METHOXY-4,5-DIHYDRO-1H-BENZO[B]AZEPIN-2(3H)-ONE structure](https://image.chemsrc.com/caspic/252/22246-83-9.png) CAS#:22246-83-9
CAS#:22246-83-9![8-Methoxy-2,3,4,5-tetrahydro-benzo[c]azepin-1-one structure](https://image.chemsrc.com/caspic/035/22246-71-5.png) CAS#:22246-71-5
CAS#:22246-71-5 CAS#:19393-87-4
CAS#:19393-87-4 CAS#:19307-13-2
CAS#:19307-13-2 CAS#:30021-91-1
CAS#:30021-91-1 CAS#:26593-50-0
CAS#:26593-50-0 CAS#:2800-94-4
CAS#:2800-94-4 CAS#:861960-34-1
CAS#:861960-34-1
