7-Methoxy-3,4-dihydronaphthalen-2(1H)-one

7-Methoxy-3,4-dihydronaphthalen-2(1H)-one structure
|
Common Name | 7-Methoxy-3,4-dihydronaphthalen-2(1H)-one | ||
|---|---|---|---|---|
| CAS Number | 4133-34-0 | Molecular Weight | 176.212 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 306.8±0.0 °C at 760 mmHg | |
| Molecular Formula | C11H12O2 | Melting Point | 27 - 28ºC | |
| MSDS | Chinese USA | Flash Point | 150.8±21.4 °C | |
| Name | 7-Methoxy-2-tetralone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 306.8±0.0 °C at 760 mmHg |
| Melting Point | 27 - 28ºC |
| Molecular Formula | C11H12O2 |
| Molecular Weight | 176.212 |
| Flash Point | 150.8±21.4 °C |
| Exact Mass | 176.083725 |
| PSA | 26.30000 |
| LogP | 1.64 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.549 |
| InChIKey | XEAPZXNZOJGVCZ-UHFFFAOYSA-N |
| SMILES | COc1ccc2c(c1)CC(=O)CC2 |
| Storage condition | 0-6°C |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Synthesis and dopaminergic activity of 2-substituted octahydrobenzo[f]quinolines.
J. Med. Chem. 32 , 961, (1989) A series of 2-substituted octahydrobenzo[f]quinolines has been synthesized and assayed for dopamine agonist activity. Only the compounds corresponding to the beta-rotameric conformation of dopamine sh... |
|
|
8,9-methylenedioxybenzo[i]phenanthridines: topoisomerase I-targeting activity and cytotoxicity.
Bioorg. Med. Chem. 11(17) , 3795-3805, (2003) Substituted benzo[i]phenanthridines that have incorporated within their structure an 8,9-methylenedioxy group can exhibit topoisomerase I-targeting activity. Structure-activity studies were performed ... |
| 3,4-Dihydro-7-methoxy-2(1H)-naphthalenone |
| 3,4-dihydro-7-methoxynaphthalen-1(2H)-one |
| 7-Methoxy-3,4-dihydronaphthalen-1(2H)-one |
| EINECS 223-954-1 |
| MFCD00001730 |
| 7-Methoxy-2-tetralone |
| 1(2H)-Naphthalenone, 3,4-dihydro-7-methoxy- |
| 2(1H)-Naphthalenone, 3,4-dihydro-7-methoxy- |
| 7-methoxy-3,4-dihydro-1H-naphthalen-2-one |
| 7-Methoxy-3,4-dihydro-2(1H)-naphthalenone |
| 7-Methoxy-3,4-dihydronaphthalen-2(1H)-one |
| 7-Methoxy-3,4-dihydro-1(2H)-naphthalenone |
 CAS#:1048330-08-0
CAS#:1048330-08-0 CAS#:1190847-93-8
CAS#:1190847-93-8![6-METHOXY-1A,2,3,7B-TETRAHYDRO-1-OXA-CYCLOPROPA[A]NAPHTHALENE Structure](https://image.chemsrc.com/caspic/339/63320-02-5.png) CAS#:63320-02-5
CAS#:63320-02-5 CAS#:60-29-7
CAS#:60-29-7 CAS#:3469-26-9
CAS#:3469-26-9 CAS#:153993-00-1
CAS#:153993-00-1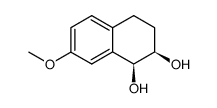 CAS#:75804-69-2
CAS#:75804-69-2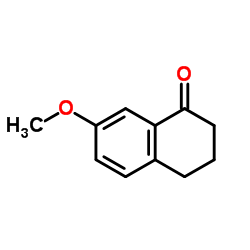 CAS#:6836-19-7
CAS#:6836-19-7 CAS#:123-11-5
CAS#:123-11-5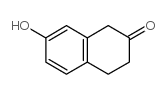 CAS#:37827-68-2
CAS#:37827-68-2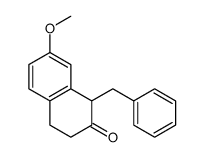 CAS#:263714-29-0
CAS#:263714-29-0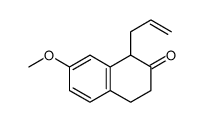 CAS#:29093-46-7
CAS#:29093-46-7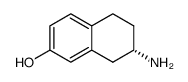 CAS#:85951-60-6
CAS#:85951-60-6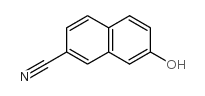 CAS#:130200-58-7
CAS#:130200-58-7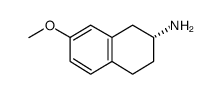 CAS#:121216-43-1
CAS#:121216-43-1 CAS#:121216-42-0
CAS#:121216-42-0 CAS#:35485-66-6
CAS#:35485-66-6![2,3,4,5-TETRAHYDRO-7-METHOXY-1H-BENZO[D]AZEPINE structure](https://image.chemsrc.com/caspic/245/50351-80-9.png) CAS#:50351-80-9
CAS#:50351-80-9 CAS#:247133-22-8
CAS#:247133-22-8
