5-Methylindole
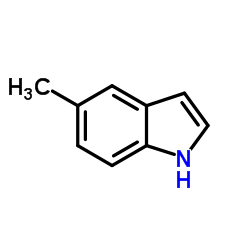
5-Methylindole structure
|
Common Name | 5-Methylindole | ||
|---|---|---|---|---|
| CAS Number | 614-96-0 | Molecular Weight | 131.17 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 269.0±9.0 °C at 760 mmHg | |
| Molecular Formula | C9H9N | Melting Point | 60-62 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 114.7±11.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 5-Methylindole5-Methylindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 5-Methylindole |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Methylindole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 269.0±9.0 °C at 760 mmHg |
| Melting Point | 60-62 °C(lit.) |
| Molecular Formula | C9H9N |
| Molecular Weight | 131.17 |
| Flash Point | 114.7±11.3 °C |
| Exact Mass | 131.073502 |
| PSA | 15.79000 |
| LogP | 2.60 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.655 |
| InChIKey | YPKBCLZFIYBSHK-UHFFFAOYSA-N |
| SMILES | Cc1ccc2[nH]ccc2c1 |
| Storage condition | Keep Cold |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
NMR studies of the mode of binding of corepressors and inducers to Escherichia coli trp repressor.
Eur. J. Biochem. 235(3) , 804-13, (1996) The binding of the corepressors tryptophan and 5-methyltryptophan and of the inducers 3-indolepropionate, 3-indoleacrylate and 5-methylindole to the Escherichia coli trp repressor have been studied by... |
|
|
Generation of new protein kinase inhibitors utilizing cytochrome p450 mutant enzymes for indigoid synthesis.
J. Med. Chem. 47(12) , 3236-41, (2004) Indigoids, a class of bis-indoles, represent a promising protein kinase inhibitor scaffold. Oxidation of indole by cytochrome P450 (P450) has been shown to generate species (indoxyl, isatin) that coup... |
|
|
Mass analyzed threshold ionization spectroscopy of 5-methylindole and 3-methylindole cations and the methyl substitution effect.
J. Chem. Phys. 120(11) , 5057-63, (2004) The vibrationally resolved mass analyzed threshold ionization spectra of jetcooled 5-methylindole (5MI) and 3-methylindole (3MI) have been recorded by ionizing via various vibronic levels of each spec... |
| 6-methylindole |
| 5-methyl-indole |
| 5-Methylindole |
| 5-methyl indane-1,3-dione |
| 5-Methylindan-1,3-dion |
| 5-Methyl-1H-indole |
| 5-metyl-1H-indole |
| Indole, 5-methyl- |
| MFCD00005680 |
| 1H-Indole, 5-methyl- |
| 5-Methylindol |
| EINECS 210-400-9 |
| 5-methyl indan-1,3-dione |
 CAS#:215589-37-0
CAS#:215589-37-0 CAS#:52562-50-2
CAS#:52562-50-2 CAS#:126759-32-8
CAS#:126759-32-8 CAS#:637-39-8
CAS#:637-39-8 CAS#:106-49-0
CAS#:106-49-0 CAS#:102-71-6
CAS#:102-71-6 CAS#:107734-14-5
CAS#:107734-14-5 CAS#:65826-95-1
CAS#:65826-95-1 CAS#:180624-14-0
CAS#:180624-14-0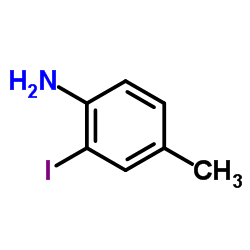 CAS#:29289-13-2
CAS#:29289-13-2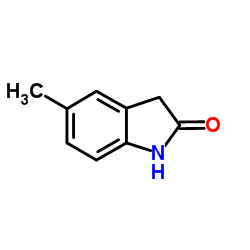 CAS#:3484-35-3
CAS#:3484-35-3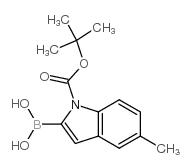 CAS#:475102-14-8
CAS#:475102-14-8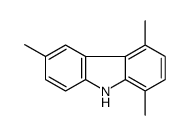 CAS#:18028-56-3
CAS#:18028-56-3 CAS#:18210-83-8
CAS#:18210-83-8 CAS#:4692-99-3
CAS#:4692-99-3 CAS#:1912-47-6
CAS#:1912-47-6 CAS#:1003708-62-0
CAS#:1003708-62-0
