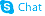CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UY9100000
-
CHEMICAL NAME :
-
7H-Pyrrolo(2,3-d)pyrimidine-5-carbonitrile, 4-amino-7-beta-D-ribofuranosyl-
-
CAS REGISTRY NUMBER :
-
606-58-6
-
BEILSTEIN REFERENCE NO. :
-
0048454
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
8
-
MOLECULAR FORMULA :
-
C12-H13-N5-O4
-
MOLECULAR WEIGHT :
-
291.30
-
WISWESSER LINE NOTATION :
-
T56 BN GN INJ DCN FZ B- BT5OTJ CQ DQ E1Q
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
8 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
20 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1500 ug/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
MUTATION DATA
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TEST SYSTEM :
-
Rodent - mouse Leukocyte
-
DOSE/DURATION :
-
71 ug/L
-
REFERENCE :
-
JMCMAR Journal of Medicinal Chemistry. (American Chemical Soc., Distribution Office Dept. 223, POB POB 57136, West End Stn., Washington, DC 20037) V.6- 1963- Volume(issue)/page/year: 30,481,1987
|

![5-chloro-9-[3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-2,4,9-triazabicyclo[4.3.0]nona-2,4,7,10-tetraene-7-carbonitrile structure](https://www.chemsrc.com/caspic/495/52443-16-0.png) CAS#:52443-16-0
CAS#:52443-16-0