| Structure | Name/CAS No. | Articles |
|---|---|---|
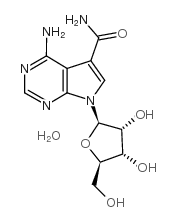 |
Sangivamycin
CAS:18417-89-5 |
|
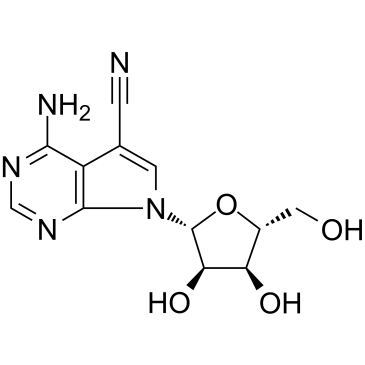 |
Toyocamycin
CAS:606-58-6 |