| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
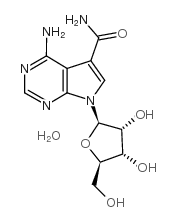 |
NSC 65346
CAS:18417-89-5 |
|
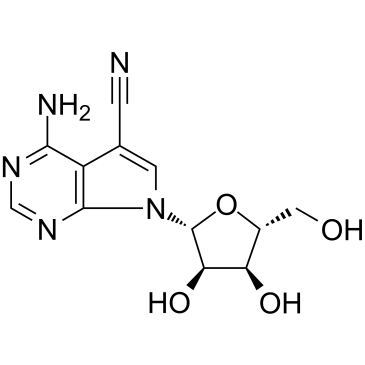 |
丰加霉素
CAS:606-58-6 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
NSC 65346
CAS:18417-89-5 |
|
 |
丰加霉素
CAS:606-58-6 |