Synthesis and in vitro antitumor activity of some amino-deoxy 7-hexofuranosylpyrrolo[2,3-d]pyrimidines.
B G Huang, M Bobek
Index: Carbohydr. Res. 308(3-4) , 319-28, (1998)
Full Text: HTML
Abstract
7-(6-amino-6-deoxy-beta-D-glucofuranosyl)-5-cyanopyrrolo[2,3 -d]pyrimidine (22) and 7-(3-amino-methyl-3-deoxy-beta-D-allofuranosyl)-5- cyanopyrrolo[2,3-d]pyrimidine (28) were synthesized by sequentially coupling silylated 4-amino-6-bromo-5- cyanopyrrolo[2,3-d]pyrimidine with the corresponding protected sugars 9 and 17, followed by deblocking and catalytic hydrogenation. Conversion of the 5-nitrile in 22 and 28 into a carboxamide gave the corresponding sangivamycin derivatives 23 and 29. Whereas 5'-aminomethyl nucleosides 22 and 23 inhibited the growth of four different human tumor cell lines at microM concentrations, the 3'-aminomethyl analogs 28 and 29 were much less active against these cells.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
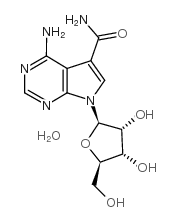 |
Sangivamycin
CAS:18417-89-5 |
C12H15N5O5 |
|
Kinetics and localization of the phosphorylation of rhodopsi...
1995-03-24 [J. Biol. Chem. 270(12) , 6710-7, (1995)] |
|
Synthesis of pyrrolo[2,1-f][1,2,4]triazine C-nucleosides. Is...
2001-03-09 [Carbohydr. Res. 331(1) , 77-82, (2001)] |
|
Shape changes and chemokinesis of Walker carcinosarcoma cell...
1993-01-01 [Anticancer Res. 13(2) , 347-54, (1993)] |
|
Sangivamycin-like molecule 6 exhibits potent anti-multiple m...
2012-11-01 [Mol. Cancer Ther. 11(11) , 2321-30, (2012)] |
|
Prolonged reversal of morphine tolerance with no reversal of...
2002-12-20 [Brain Res. 958(1) , 28-35, (2002)] |