Phloretin
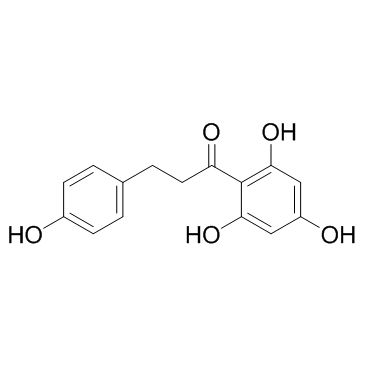
Phloretin structure
|
Common Name | Phloretin | ||
|---|---|---|---|---|
| CAS Number | 60-82-2 | Molecular Weight | 274.269 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 534.4±29.0 °C at 760 mmHg | |
| Molecular Formula | C15H14O5 | Melting Point | ~260 °C | |
| MSDS | Chinese USA | Flash Point | 291.1±20.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of PhloretinPhloretin(NSC 407292; RJC 02792) is a dihydrochalcone, a type of natural phenols. Phloretin inhibits the active transport of glucose into cells by SGLT1 and SGLT2.IC50 Value: 49 +/- 12 microM [4]Target: SGLT1/2in vitro: Phlorizin blocks glucose transport across the renal tubule at concentrations in renal blood and tissue in the range of 10-5 to 10-7 M [1]. PT significantly enhanced glycerol release and inhibited the adipogenesis-related transcription factors. PT also promoted phosphorylation of AMP-activated protein kinase and increased activity of adipose triglyceride lipase and hormone-sensitive lipase in 3T3-L1 cells [2]. Phloretin induced obvious cytotoxicity against BEL-7402 cells with IC50 of 89.23 microg/mL. The growth curve demonstrated decreased growth of the cells as phloretin concentration increased [3]. D-glucose-transport activity was observed with a Km for D-glucose of 3.4 +/- 0.2 mM (mean +/- S.E.M.) and was inhibited by cytochalasin B (IC50= 0.44 +/- 0.03 microM), HgCl2 (IC50)= 3.5 +/- 0.5 microM), phloretin (IC50= 49 +/- 12 microM) and phloridzin (IC50= 355 +/- 67 microM) [4].in vivo: The effect of phloridzin orally doses 5, 10, 20 and 40 mg/kg body weight on diabetes was tested in a streptozotocin-induced rat model of diabetes type 1. From beneficial effect of this compound is significant reduction of blood glucose levels and improve dyslipidemia in diabetic rats [5]. |
| Name | phloretin |
|---|---|
| Synonym | More Synonyms |
| Description | Phloretin(NSC 407292; RJC 02792) is a dihydrochalcone, a type of natural phenols. Phloretin inhibits the active transport of glucose into cells by SGLT1 and SGLT2.IC50 Value: 49 +/- 12 microM [4]Target: SGLT1/2in vitro: Phlorizin blocks glucose transport across the renal tubule at concentrations in renal blood and tissue in the range of 10-5 to 10-7 M [1]. PT significantly enhanced glycerol release and inhibited the adipogenesis-related transcription factors. PT also promoted phosphorylation of AMP-activated protein kinase and increased activity of adipose triglyceride lipase and hormone-sensitive lipase in 3T3-L1 cells [2]. Phloretin induced obvious cytotoxicity against BEL-7402 cells with IC50 of 89.23 microg/mL. The growth curve demonstrated decreased growth of the cells as phloretin concentration increased [3]. D-glucose-transport activity was observed with a Km for D-glucose of 3.4 +/- 0.2 mM (mean +/- S.E.M.) and was inhibited by cytochalasin B (IC50= 0.44 +/- 0.03 microM), HgCl2 (IC50)= 3.5 +/- 0.5 microM), phloretin (IC50= 49 +/- 12 microM) and phloridzin (IC50= 355 +/- 67 microM) [4].in vivo: The effect of phloridzin orally doses 5, 10, 20 and 40 mg/kg body weight on diabetes was tested in a streptozotocin-induced rat model of diabetes type 1. From beneficial effect of this compound is significant reduction of blood glucose levels and improve dyslipidemia in diabetic rats [5]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.4±29.0 °C at 760 mmHg |
| Melting Point | ~260 °C |
| Molecular Formula | C15H14O5 |
| Molecular Weight | 274.269 |
| Flash Point | 291.1±20.8 °C |
| Exact Mass | 274.084137 |
| PSA | 97.99000 |
| LogP | 3.50 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.685 |
| InChIKey | VGEREEWJJVICBM-UHFFFAOYSA-N |
| SMILES | O=C(CCc1ccc(O)cc1)c1c(O)cc(O)cc1O |
| Storage condition | 2-8°C |
| Water Solubility | soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2942000000 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Targeting metabolic flexibility by simultaneously inhibiting respiratory complex I and lactate generation retards melanoma progression.
Oncotarget 6 , 37281-99, (2015) Melanoma is a largely incurable skin malignancy owing to the underlying molecular and metabolic heterogeneity confounded by the development of resistance. Cancer cells have metabolic flexibility in ch... |
|
|
Antagonistic control of a dual-input mammalian gene switch by food additives.
Nucleic Acids Res. 42(14) , e116, (2014) Synthetic biology has significantly advanced the design of mammalian trigger-inducible transgene-control devices that are able to programme complex cellular behaviour. Fruit-based benzoate derivatives... |
|
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
| QR CQ EQ BV2R DQ |
| 1-Propanone, 3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)- |
| Dihydronaringenin |
| 2',4',6',4-tetrahydroxydihydrochalcone |
| 3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone |
| β-(p-Hydroxyphenyl)phloropropiophenone |
| phloretol |
| 4,2',4',6'-tetrahydroxydihydrochalcone |
| b-(p-Hydroxyphenyl)phloropropiophenone |
| UNII-S5J5OE47MK |
| 3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one |
| EINECS 200-488-7 |
| Phloretin |
| MFCD00002288 |
 CAS#:480-41-1
CAS#:480-41-1 CAS#:60-81-1
CAS#:60-81-1 CAS#:25515-46-2
CAS#:25515-46-2 CAS#:88607-79-8
CAS#:88607-79-8 CAS#:108-73-6
CAS#:108-73-6 CAS#:501-97-3
CAS#:501-97-3 CAS#:4397-53-9
CAS#:4397-53-9![1-[2,4-Bis(benzyloxy)-6-hydroxyphenyl]ethanone Structure](https://image.chemsrc.com/caspic/002/18065-05-9.png) CAS#:18065-05-9
CAS#:18065-05-9 CAS#:524-14-1
CAS#:524-14-1 CAS#:18916-17-1
CAS#:18916-17-1 CAS#:107585-77-3
CAS#:107585-77-3 CAS#:10210-17-0
CAS#:10210-17-0 CAS#:108-46-3
CAS#:108-46-3 CAS#:99-96-7
CAS#:99-96-7![5,7-dihydroxy-3-[(4-hydroxyphenyl)methyl]chromen-4-one structure](https://image.chemsrc.com/caspic/261/101467-70-3.png) CAS#:101467-70-3
CAS#:101467-70-3 CAS#:76344-03-1
CAS#:76344-03-1![[3,5-diacetyloxy-2-[3-(4-acetyloxyphenyl)propanoyl]phenyl] acetate structure](https://image.chemsrc.com/caspic/118/42385-89-7.png) CAS#:42385-89-7
CAS#:42385-89-7 CAS#:3791-75-1
CAS#:3791-75-1
