Synephrine hydrochloride
Modify Date: 2025-08-25 16:25:16
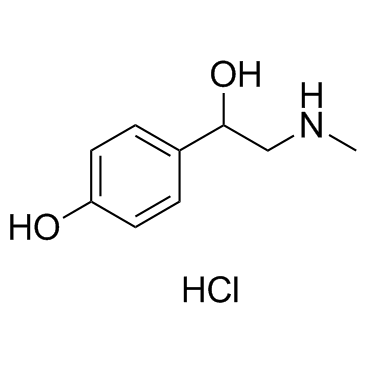
Synephrine hydrochloride structure
|
Common Name | Synephrine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 5985-28-4 | Molecular Weight | 203.666 | |
| Density | N/A | Boiling Point | 341.1ºC at 760 mmHg | |
| Molecular Formula | C9H14ClNO2 | Melting Point | 147-150ºC | |
| MSDS | N/A | Flash Point | 163.4ºC | |
Use of Synephrine hydrochlorideSynephrine Hcl(Oxedrine) is an alkaloid; synephrine produces most of its biological effects by acting as an agonist at adrenergic receptors.IC50 value:Target: adrenergic receptor agonistThere is some evidence that synephrine also has weak activity at 5-HT receptors, and that it interacts with TAAR1 (trace adrenergic amine receptors). d-synephrine inhibited the uptake of [3H]-norepinephrine with an IC50 = 5.8 μM; l-synephrine was less potent (IC50 = 13.5 μM). d-Synephrine also competitively inhibited the binding of nisoxetine[m] to rat brain cortical slices, with a Ki = 4.5 μM; l-synephrine was less potent (Ki = 8.2 μM). In experiments on the release of [3H]-norepinephrine from rat brain cortical slices, however, the l-isomer of synephrine was a more potent enhancer of the release (EC50 = 8.2 μM) than the d-isomer (EC50 = 12.3 μM). This enhanced release by l-synephrine was blocked by nisoxetine. |
| Name | 4-[1-hydroxy-2-(methylamino)ethyl]phenol,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Synephrine Hcl(Oxedrine) is an alkaloid; synephrine produces most of its biological effects by acting as an agonist at adrenergic receptors.IC50 value:Target: adrenergic receptor agonistThere is some evidence that synephrine also has weak activity at 5-HT receptors, and that it interacts with TAAR1 (trace adrenergic amine receptors). d-synephrine inhibited the uptake of [3H]-norepinephrine with an IC50 = 5.8 μM; l-synephrine was less potent (IC50 = 13.5 μM). d-Synephrine also competitively inhibited the binding of nisoxetine[m] to rat brain cortical slices, with a Ki = 4.5 μM; l-synephrine was less potent (Ki = 8.2 μM). In experiments on the release of [3H]-norepinephrine from rat brain cortical slices, however, the l-isomer of synephrine was a more potent enhancer of the release (EC50 = 8.2 μM) than the d-isomer (EC50 = 12.3 μM). This enhanced release by l-synephrine was blocked by nisoxetine. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 341.1ºC at 760 mmHg |
|---|---|
| Melting Point | 147-150ºC |
| Molecular Formula | C9H14ClNO2 |
| Molecular Weight | 203.666 |
| Flash Point | 163.4ºC |
| Exact Mass | 203.071304 |
| PSA | 52.49000 |
| LogP | 1.83790 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2922199090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| UNII:EN5D1IH09S |
| DL-synephrine hydrochloride |
| EINECS 227-804-6 |
| Synephrine HCl |
| 4-(1-Hydroxy-2-(methylamino)ethyl)phenol hydrochloride |
| MFCD00050566 |
| Synephrine hydrochloride |
| 4-[1-Hydroxy-2-(methylamino)ethyl]phenol hydrochloride (1:1) |
| 2-methylamino-1-(4-hydroxyphenyl)-ethanol hydrochloride |
| Benzenemethanol, 4-hydroxy-α-[(methylamino)methyl]-, hydrochloride (1:1) |
| (+-)-1-(4-hydroxy-phenyl)-2-methylamino-ethanol,hydrochloride |
| Oxedrine hydrochloride |
| Ocuton (TN) |
| 1-(4-Hydroxy-phenyl)-2-methylamino-aethanol,Hydrochlorid |
 CAS#:14593-25-0
CAS#:14593-25-0
