pyrimethamine
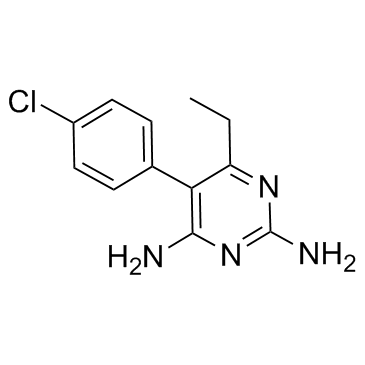
pyrimethamine structure
|
Common Name | pyrimethamine | ||
|---|---|---|---|---|
| CAS Number | 58-14-0 | Molecular Weight | 248.711 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 368.4±52.0 °C at 760 mmHg | |
| Molecular Formula | C12H13ClN4 | Melting Point | 233-234°C | |
| MSDS | Chinese USA | Flash Point | 176.6±30.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of pyrimethaminePyrimethamine(RP4753) is a medication used for protozoal infections; interferes with tetrahydrofolic acid synthesis from folic acid by inhibiting the enzyme dihydrofolate reductase (DHFR).IC50 Value: 15.4 nM (Plasmodium falciparum) [1]Target: DHFR; antifolatein vitro: Three susceptibility levels (susceptible, intermediate, and resistant) were observed in the response of culture-adapted clones and strains to pyrimethamine (50% inhibitory concentration [IC50]) < 100, 100-2,000, and > 2,000 nM) and cycloguanil (IC50 < 50, 50-500, and > 500 nM). Based on these susceptibility levels, 73 and 68 of 96 fresh clinical isolates were susceptible to pyrimethamine (mean IC50 15.4 nM) and cycloguanil (mean IC50 11.1 nM), respectively [1]. We tested pyrimethamine(previously reported to suppress SOD1 expression), several compounds currently in trials in human and murine ALS, and a set of 1040 FDA-approved compounds. In a PC12 cell-based assay, no compounds reduced SOD1 promoter activity without concomitant cytotoxicity. Additionally,pyrimethamine failed to repress levels of SOD1 protein in HeLa cells or homogenates of liver, spinal cord and brain of wild-type mice [3].in vivo: (131)I-Pyrimethamine (specific activity: 7.08 MBq/ mol) was injected intravenously into the tail vein of the control and infected rats. Static whole body images of the rats were acquired under the gamma camera at 5 min, 45 min, 2 h, 6 h, and 24 h following the intravenous administration of the radioactivity (3.7 MBq/rat) [2]. The 10-day treatment with 10mg/kg/day of fluconazole combined with 40/1mg/kg/day sulfadiazine and pyrimethamine resulted in 93% survival of CF1 mice acutely infected with the highly virulent T. gondii RH strain, versus 36% of mice treated with just sulfadiazine and pyrimethamine [4].Toxicity: Sulfadoxine/pyrimethamine is well tolerated as treatment and when used as intermittent preventive treatment in pregnant African women. Sulfadoxine/pyrimethamine is no longer used as prophylaxis because it may cause toxic epidermal necrolysis and Stevens Johnson syndrome [5]. |
| Name | pyrimethamine |
|---|---|
| Synonym | More Synonyms |
| Description | Pyrimethamine(RP4753) is a medication used for protozoal infections; interferes with tetrahydrofolic acid synthesis from folic acid by inhibiting the enzyme dihydrofolate reductase (DHFR).IC50 Value: 15.4 nM (Plasmodium falciparum) [1]Target: DHFR; antifolatein vitro: Three susceptibility levels (susceptible, intermediate, and resistant) were observed in the response of culture-adapted clones and strains to pyrimethamine (50% inhibitory concentration [IC50]) < 100, 100-2,000, and > 2,000 nM) and cycloguanil (IC50 < 50, 50-500, and > 500 nM). Based on these susceptibility levels, 73 and 68 of 96 fresh clinical isolates were susceptible to pyrimethamine (mean IC50 15.4 nM) and cycloguanil (mean IC50 11.1 nM), respectively [1]. We tested pyrimethamine(previously reported to suppress SOD1 expression), several compounds currently in trials in human and murine ALS, and a set of 1040 FDA-approved compounds. In a PC12 cell-based assay, no compounds reduced SOD1 promoter activity without concomitant cytotoxicity. Additionally,pyrimethamine failed to repress levels of SOD1 protein in HeLa cells or homogenates of liver, spinal cord and brain of wild-type mice [3].in vivo: (131)I-Pyrimethamine (specific activity: 7.08 MBq/ mol) was injected intravenously into the tail vein of the control and infected rats. Static whole body images of the rats were acquired under the gamma camera at 5 min, 45 min, 2 h, 6 h, and 24 h following the intravenous administration of the radioactivity (3.7 MBq/rat) [2]. The 10-day treatment with 10mg/kg/day of fluconazole combined with 40/1mg/kg/day sulfadiazine and pyrimethamine resulted in 93% survival of CF1 mice acutely infected with the highly virulent T. gondii RH strain, versus 36% of mice treated with just sulfadiazine and pyrimethamine [4].Toxicity: Sulfadoxine/pyrimethamine is well tolerated as treatment and when used as intermittent preventive treatment in pregnant African women. Sulfadoxine/pyrimethamine is no longer used as prophylaxis because it may cause toxic epidermal necrolysis and Stevens Johnson syndrome [5]. |
|---|---|
| Related Catalog | |
| References |
[5]. Taylor WR, et al. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25-61. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 368.4±52.0 °C at 760 mmHg |
| Melting Point | 233-234°C |
| Molecular Formula | C12H13ClN4 |
| Molecular Weight | 248.711 |
| Flash Point | 176.6±30.7 °C |
| Exact Mass | 248.082870 |
| PSA | 77.82000 |
| LogP | 1.03 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.667 |
| InChIKey | WKSAUQYGYAYLPV-UHFFFAOYSA-N |
| SMILES | CCc1nc(N)nc(N)c1-c1ccc(Cl)cc1 |
| Storage condition | 0-6°C |
| Stability | Stable, but light sensitive. Combustible. Incompatible with strong oxidizing agents. |
| Water Solubility | <0.01 g/100 mL at 21 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | UV8140000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 10 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Multiresidue method for the determination of pharmacologically active substances in egg and honey using a continuous solid-phase extraction system and gas chromatography-mass spectrometry.
Food Chem. 178 , 63-9, (2015) A sensitive, selective, efficient gas chromatography-mass spectrometry method for the simultaneous determination of 22 pharmacologically active substances (antibacterials, nonsteroidal antiinflammator... |
|
|
Host erythrocyte environment influences the localization of exported protein 2, an essential component of the Plasmodium translocon.
Eukaryotic Cell 14(4) , 371-84, (2015) Malaria parasites replicating inside red blood cells (RBCs) export a large subset of proteins into the erythrocyte cytoplasm to facilitate parasite growth and survival. PTEX, the parasite-encoded tran... |
|
|
Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria.
PLoS Pathog. 11(3) , e1004715, (2015) Interleukin-21 signaling is important for germinal center B-cell responses, isotype switching and generation of memory B cells. However, a role for IL-21 in antibody-mediated protection against pathog... |
| Daraclor |
| Pyremethamine |
| Primethamine |
| 5-(4-Chlorophenyl)-6-ethyl-2,4-pyrimidinediamine |
| TCMDC-125860 |
| WR 2978 |
| Pyrimethamin |
| Malocid |
| pirimetamin |
| bw50-63 |
| 5-(4-chlorophényl)-6-éthylpyrimidine-2,4-diamine |
| Malacid |
| 2,4-Pyrimidinediamine, 5-(4-chlorophenyl)-6-ethyl- |
| Daraprim |
| Malocide |
| 5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |
| 5-(4-Chlorphenyl)-6-ethylpyrimidin-2,4-diamin |
| MFCD00057350 |
| T6N CNJ BZ DZ ER DG& F2 |
| RP 4753 |
| chloridine |
| darachlor |
| pirimecidan |
| Pyrimethamine |
| Pirimetamina |
| EINECS 200-364-2 |
| TCMDC-123831 |
| 4753r.p. |
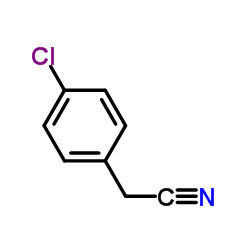 CAS#:140-53-4
CAS#:140-53-4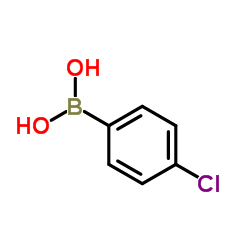 CAS#:1679-18-1
CAS#:1679-18-1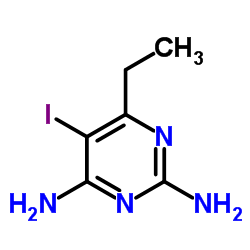 CAS#:514854-13-8
CAS#:514854-13-8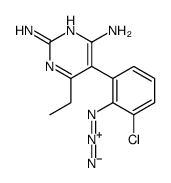 CAS#:95458-40-5
CAS#:95458-40-5 CAS#:186581-53-3
CAS#:186581-53-3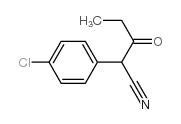 CAS#:55474-40-3
CAS#:55474-40-3![N-[4-ethyl-6-chloro-5-(4-chloro-phenyl)-pyrimidin-2-yl]-acetamide Structure](https://image.chemsrc.com/caspic/459/100716-90-3.png) CAS#:100716-90-3
CAS#:100716-90-3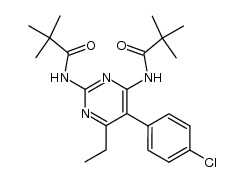 CAS#:143947-40-4
CAS#:143947-40-4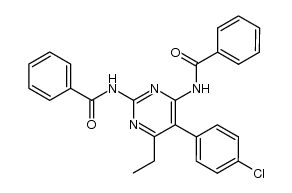 CAS#:143947-41-5
CAS#:143947-41-5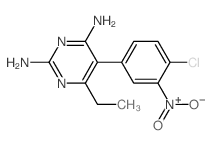 CAS#:21813-35-4
CAS#:21813-35-4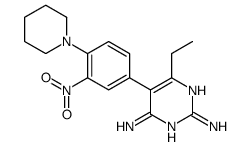 CAS#:104484-42-6
CAS#:104484-42-6![N-[2-chloro-5-(2,4-diamino-6-ethylpyrimidin-5-yl)phenyl]acetamide structure](https://image.chemsrc.com/caspic/214/113338-16-2.png) CAS#:113338-16-2
CAS#:113338-16-2![N'-[2-chloro-5-(2,4-diamino-6-ethylpyrimidin-5-yl)phenyl]-N,N-dimethylmethanimidamide structure](https://image.chemsrc.com/caspic/315/113494-46-5.png) CAS#:113494-46-5
CAS#:113494-46-5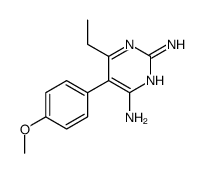 CAS#:113512-19-9
CAS#:113512-19-9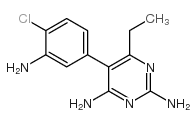 CAS#:24851-19-2
CAS#:24851-19-2![N-[4-amino-5-(4-chlorophenyl)-6-ethylpyrimidin-2-yl]acetamide structure](https://image.chemsrc.com/caspic/427/74124-11-1.png) CAS#:74124-11-1
CAS#:74124-11-1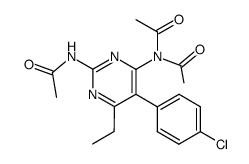 CAS#:74124-12-2
CAS#:74124-12-2
