Protoporphyrin IX
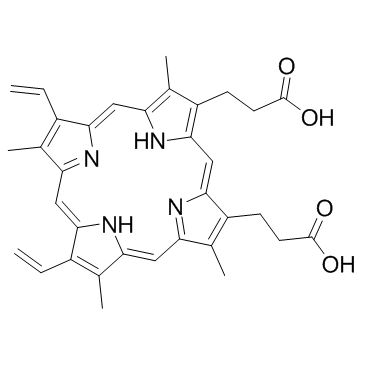
Protoporphyrin IX structure
|
Common Name | Protoporphyrin IX | ||
|---|---|---|---|---|
| CAS Number | 553-12-8 | Molecular Weight | 562.658 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 1122.0±65.0 °C at 760 mmHg | |
| Molecular Formula | C34H34N4O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 632.4±34.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Protoporphyrin IXProtoporphyrin IX is the final intermediate in the heme biosynthetic pathway. |
| Name | protoporphyrin |
|---|---|
| Synonym | More Synonyms |
| Description | Protoporphyrin IX is the final intermediate in the heme biosynthetic pathway. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1122.0±65.0 °C at 760 mmHg |
| Molecular Formula | C34H34N4O4 |
| Molecular Weight | 562.658 |
| Flash Point | 632.4±34.3 °C |
| Exact Mass | 562.257996 |
| PSA | 130.90000 |
| LogP | 7.33 |
| Appearance of Characters | Powder | purple |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P280-P305 + P351 + P338-P337 + P313 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26;S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 6 | |
|---|---|
| DownStream 10 | |
|
Retinoic acid synergizes ATO-mediated cytotoxicity by precluding Nrf2 activity in AML cells.
Br. J. Cancer 111(5) , 874-82, (2014) Standard therapy for acute promyelocytic leukaemia (APL) includes retinoic acid (all-trans retinoic acid (ATRA)), which promotes differentiation of promyelocytic blasts. Although co-administration of ... |
|
|
Indomethacin inhibits activation of endothelial nitric oxide synthase in the rat kidney: possible role of this effect in the pathogenesis of indomethacin-induced renal damage.
Chem. Biol. Interact. 221 , 77-87, (2014) The clinical use of non-steroidal anti-inflammatory drugs (NSAIDs) is often associated with adverse effects in the kidney. Indomethacin, an NSAID that has been shown to induce oxidative stress in the ... |
|
|
Comparison of protoporphyrin IX produced cell proliferation inhibition between human breast cancer MCF-7 and MDA-MB-231 cells.
Pharmazie 69(8) , 621-8, (2014) Protoporphyrin IX (PpIX) is an effective hematoporphyrin derivative, widely adopted in photodynamic therapy (PDT) and sonodynamic therapy (SDT). As a sensitizer, PpIX could significantly enhance laser... |
| 3,3'-(3,7,12,17-Tetramethyl-8,13-divinyl-2,18-porphyrindiyl)dipropanoic acid |
| 3,3'-(7,12-diethenyl-3,8,13,17-tetramethylporphyrin-2,18-diyl)dipropanoic acid |
| Kammerer's prophyrin |
| 1,3,5,8-Tetramethyl-2,4-divinylporphine-6,7-dipropionic Acid |
| MFCD00151109 |
| 3,3'-(3,7,12,17-Tetramethyl-8,13-divinylporphyrin-2,18-diyl)dipropanoic acid |
| 21H,23H-Porphine-2,18-dipropanoic acid, 7,12-diethenyl-3,8,13,17-tetramethyl- |
| protoporphyrin |
| EINECS 209-033-7 |
| Kammerer's porphyrin |
| protoporphyrin-IX |
| 7,12-Diethenyl-3,8,13,17-tetramethyl-21H,23H-porphine-2,18-dipropanoic acid |
| Protoporphyrin IX |
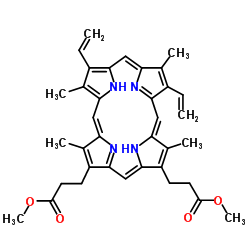 CAS#:5522-66-7
CAS#:5522-66-7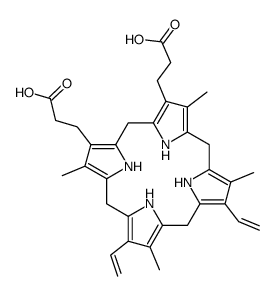 CAS#:7412-77-3
CAS#:7412-77-3![[5,5'-diformyl-4,4'-dimethyl-3,3'-bis[2-(methoxycarbonyl)ethyl]-2,2'-dipyrryl]methane Structure](https://image.chemsrc.com/caspic/146/4792-10-3.png) CAS#:4792-10-3
CAS#:4792-10-3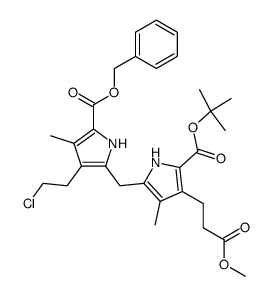 CAS#:51089-85-1
CAS#:51089-85-1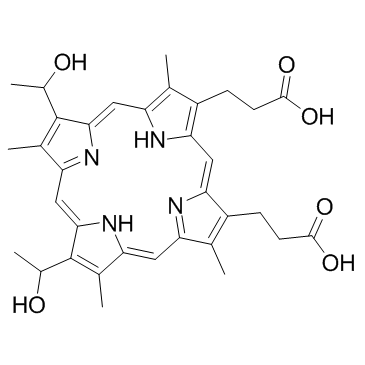 CAS#:14459-29-1
CAS#:14459-29-1![3-[8,13-diacetyl-18-(2-carboxyethyl)-3,7,12,17-tetramethyl-22,23-dihydroporphyrin-2-yl]propanoic acid Structure](https://image.chemsrc.com/caspic/087/4792-07-8.png) CAS#:4792-07-8
CAS#:4792-07-8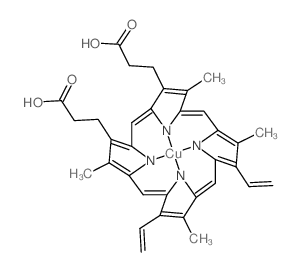 CAS#:14494-37-2
CAS#:14494-37-2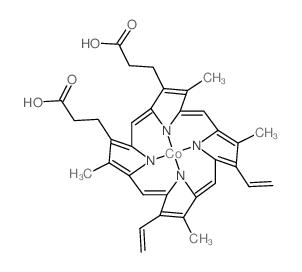 CAS#:14325-03-2
CAS#:14325-03-2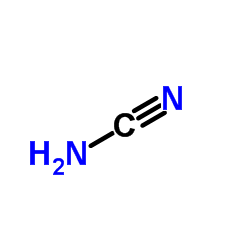 CAS#:420-04-2
CAS#:420-04-2 CAS#:7664-93-9
CAS#:7664-93-9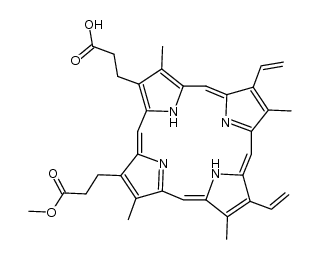 CAS#:109224-33-1
CAS#:109224-33-1 CAS#:16053-68-2
CAS#:16053-68-2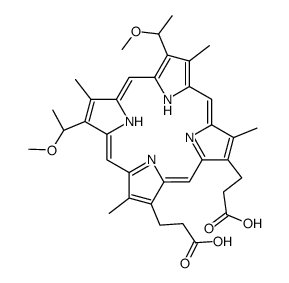 CAS#:31444-62-9
CAS#:31444-62-9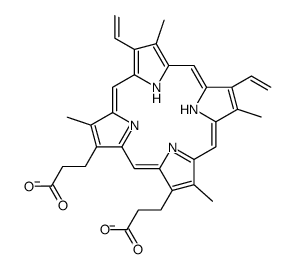 CAS#:15489-47-1
CAS#:15489-47-1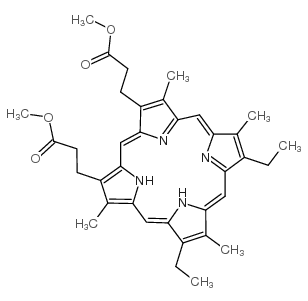 CAS#:1263-63-4
CAS#:1263-63-4
