Myricetin
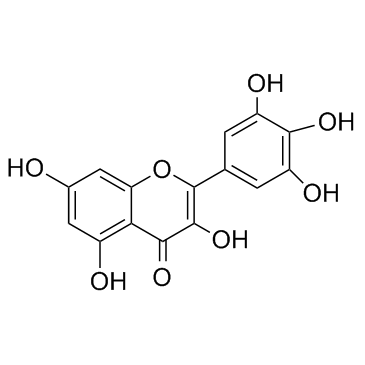
Myricetin structure
|
Common Name | Myricetin | ||
|---|---|---|---|---|
| CAS Number | 529-44-2 | Molecular Weight | 318.235 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 747.6±60.0 °C at 760 mmHg | |
| Molecular Formula | C15H10O8 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 285.9±26.4 °C | |
Use of MyricetinMyricetin is a common plant-derived flavonoid with a wide range of activities including strong anti-oxidant, anticancer, antidiabetic and anti-inflammatory activities. |
| Name | myricetin |
|---|---|
| Synonym | More Synonyms |
| Description | Myricetin is a common plant-derived flavonoid with a wide range of activities including strong anti-oxidant, anticancer, antidiabetic and anti-inflammatory activities. |
|---|---|
| Related Catalog | |
| In Vitro | Myricetin exhibits the scavenging activity towards a number of radicals and ions. It displays poor activity (IC50 value=1.4 mg/mL) in a superoxide dismutase (SOD)-like activity assay[1]. It prevents cancer cell death via apoptosis via regulation of PI3K/Akt and MAPK signalling pathways[2]. Myricetin exhibits antiphotoaging effects by quenching causative free radicals in the skin. Myricetin is able to suppress UVB-induced COX-2 expression in mouse skin epidermal JB6 P+ cells. It inhibits UVB-induced initiation of activator protein-1 and NF-κβ, as well as Fyn kinase activity[1]. Myricetin inhibits viability of SKOV3 ovarian cancer cells in a dose-dependent manner. It induces DNA DSBs and ER stress, which leads to apoptosis in SKOV3 cells[3]. Myricetin inhibits human Hsp70 by more than 80% with IC50 values of 83, 11 and 12 μM, respectively[4]. |
| In Vivo | Treatment of orthotopic pancreatic tumors with myricetin results in tumor regression and decreases metastatic spread[2]. Exposure to 150 μM myricetin causes 14%, 26%, 5% and 49% inhibition of rabbit platelet aggregation, induced by ADP, arachidonic acid, collagen and PAF, respectively[5]. |
| Cell Assay | Pancreatic cancer cells (MIA PaCa-2, Panc-1 or S2-013) or normal pancreatic ductal cells (PDCs) are treated with myricetin (12.5–200 μM). Cell viability is determined using the Dojindo Cell Counting Kit-8. Cells are seeded onto a 96-well plate at 1×104 cells per well and allowed to adhere overnight. After treatment with myricetin at various concentrations for 24 hours, 10 μL of the tetrazolium substrate is added to each well of the plate. Plates are incubated at 37°C for 1 hour, after which the absorbance at 450 nm is measured[2]. |
| Animal Admin | Mice: Mice are given daily intraperitoneal injections of myricetin (30mg/kg in the MIA PaCa-2 model and 50mg/kg in the S2-013 model) or vehicle (DMSO) for 35 days (MIA PaCa-2 model) or 18 days (S2-013 model). Ultrasound measurements are performed at regular intervals to monitor tumor growth. At the end of the in vivo experiment, tumor size is measured using calipers and tumor volume is calculated[2]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 747.6±60.0 °C at 760 mmHg |
| Melting Point | >300 °C(lit.) |
| Molecular Formula | C15H10O8 |
| Molecular Weight | 318.235 |
| Flash Point | 285.9±26.4 °C |
| Exact Mass | 318.037567 |
| PSA | 151.59000 |
| LogP | 2.11 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.864 |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Precursor 4 | |
|---|---|
| DownStream 7 | |
| HS Code | 2914501900 |
|---|---|
| Summary | 2914501900 other ketone-phenols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Effect of different aging techniques on the polysaccharide and phenolic composition and sensory characteristics of Syrah red wines fermented using different yeast strains.
Food Chem. 179 , 116-26, (2015) The effect of high levels of the polysaccharide Saccharomyces cerevisiae yeast strain (HPS) and another conventional yeast strain (FERM) on the polysaccharide and phenolic composition of Syrah red win... |
|
|
Effect of selected Saccharomyces cerevisiae yeast strains and different aging techniques on the polysaccharide and polyphenolic composition and sensorial characteristics of Cabernet Sauvignon red wines.
J. Sci. Food Agric. 95 , 2132-44, (2015) The objective of this work was to study the effect of two Saccharomyces cerevisiae yeast strains with different capabilities of polysaccharide liberation during alcoholic fermentation in addition to s... |
|
|
Rapid method for the simultaneous determination of flavonol aglycones in food using u-HPLC coupled with heating block acidic hydrolysis.
J. AOAC Int. 96(5) , 1059-64, (2013) A rapid method for the simultaneous determination of flavonol aglycones in food using ultra-high-performance LC (u-HPLC) coupled with a heating-block acidic hydrolysis method was validated in terms of... |
| Delphidenolon 1575 |
| EINECS 208-463-2 |
| MFCD00006827 |
| 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one |
| 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one |
| 3,5,7,3',4',5'-hexahydroxyflavonol |
| 2-(3,4,5-TRIHYDROXYPHENYL)-3,5,7-TRIHYDROXY-4H-1-BENZOPYRAN-4-ONE |
| Myricitin |
| Myricetin |
| Cannabiscetin |
| 3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-1-benzopyran-4-one |
| Myricetol |
| 3 3' 4' 5 5' 7-hexahydroxyflavone |
| 4H-1-Benzopyran-4-one, 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)- |
| 3,5,7,3',4',5'-Hexahydroxyflavone |
| 3,3',4',5,5',7-hexahydroxyflavone |
 CAS#:17912-87-7
CAS#:17912-87-7 CAS#:15648-86-9
CAS#:15648-86-9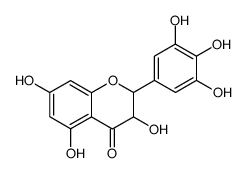 CAS#:557792-97-9
CAS#:557792-97-9 CAS#:10034-85-2
CAS#:10034-85-2 CAS#:108-73-6
CAS#:108-73-6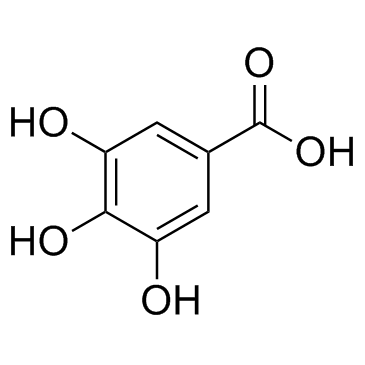 CAS#:149-91-7
CAS#:149-91-7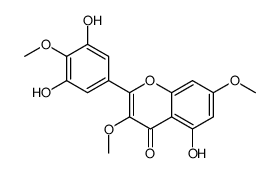 CAS#:15868-40-3
CAS#:15868-40-3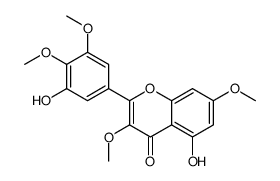 CAS#:14290-57-4
CAS#:14290-57-4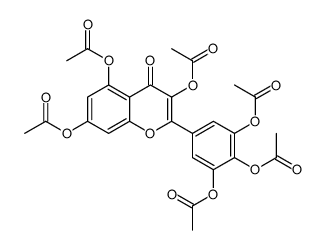 CAS#:14813-29-7
CAS#:14813-29-7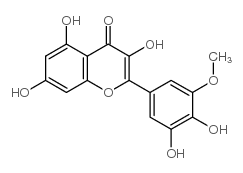 CAS#:53472-37-0
CAS#:53472-37-0
