wedelolactone
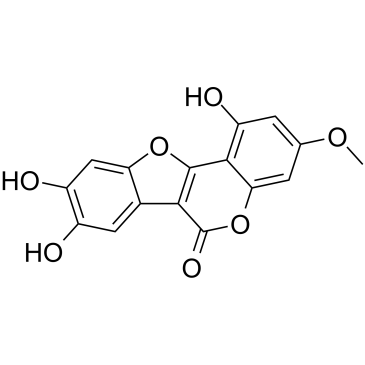
wedelolactone structure
|
Common Name | wedelolactone | ||
|---|---|---|---|---|
| CAS Number | 524-12-9 | Molecular Weight | 314.246 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 498.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C16H10O7 | Melting Point | 315 °C | |
| MSDS | Chinese USA | Flash Point | 255.2±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of wedelolactoneWedelolactone, a natural product from Ecliptae herba, suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK Complex[1]. Wedelolactone inhibits 5-lipoxygenase (5-Lox) (IC50~2.5 μM) activity by an oxygen radical scavenging mechanism. Wedelolactone induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt[2]. Anti-cancer, anti-inflammatory, and antioxidant activities[3]. |
| Name | wedelolactone |
|---|---|
| Synonym | More Synonyms |
| Description | Wedelolactone, a natural product from Ecliptae herba, suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK Complex[1]. Wedelolactone inhibits 5-lipoxygenase (5-Lox) (IC50~2.5 μM) activity by an oxygen radical scavenging mechanism. Wedelolactone induces caspase-dependent apoptosis in prostate cancer cells via downregulation of PKCε without inhibiting Akt[2]. Anti-cancer, anti-inflammatory, and antioxidant activities[3]. |
|---|---|
| Related Catalog | |
| Target |
Caspase-11 5-lipoxygenase:2.5 μM (IC50) Apoptosis |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 498.4±45.0 °C at 760 mmHg |
| Melting Point | 315 °C |
| Molecular Formula | C16H10O7 |
| Molecular Weight | 314.246 |
| Flash Point | 255.2±28.7 °C |
| Exact Mass | 314.042664 |
| PSA | 113.27000 |
| LogP | 2.75 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.759 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 14 mg/mL, soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317 |
| Precautionary Statements | P280-P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22;R43 |
| Safety Phrases | 36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Understanding the mechanisms controlling Leishmania amazonensis infection in vitro: the role of LTB4 derived from human neutrophils.
J. Infect. Dis. 210(4) , 656-66, (2014) Neutrophils are rapidly recruited to the site of Leishmania infection and play an active role in capturing and killing parasites. They are the main source of leukotriene B4 (LTB4), a potent proinflamm... |
|
|
CYLD is a crucial negative regulator of innate immune response in Escherichia coli pneumonia.
Cell. Microbiol. 10(11) , 2247-56, (2008) Bacteraemic pneumonia is a common cause of sepsis in critically ill patients today and is characterized by dysregulation of inflammation. The genetic factors predisposing to bacteraemic pneumonia are ... |
|
|
Demethylwedelolactone derivatives inhibit invasive growth in vitro and lung metastasis of MDA-MB-231 breast cancer cells in nude mice.
Eur. J. Med. Chem. 56 , 361-7, (2012) The anticancer properties of demethylwedelolactone (DWEL) and wedelolactone (WEL), which are naturally occurring coumestans, have not been well characterized. In this study, we investigated the anti-i... |
| 6H-Benzofuro[3,2-c][1]benzopyran-6-one, 1,8,9-trihydroxy-3-methoxy- |
| wedelolactone |
| 1,8,9-Trihydroxy-3-methoxy-6H-[1]benzofuro[3,2-c]chromen-6-one |
| 6H-Benzofuro(3,2-c)(1)benzopyran-6-one, 1,8,9-trihydroxy-3-methoxy- |
| 1,8,9-trihydroxy-3-methoxycoumestan |
 CAS#:627489-05-8
CAS#:627489-05-8![1-hydroxy-3-methoxy-8,9-diisopropyloxy-benzo[4,5]furo[3,2-c]chromen-6-one Structure](https://image.chemsrc.com/caspic/328/1023744-97-9.png) CAS#:1023744-97-9
CAS#:1023744-97-9 CAS#:5447-02-9
CAS#:5447-02-9 CAS#:27688-86-4
CAS#:27688-86-4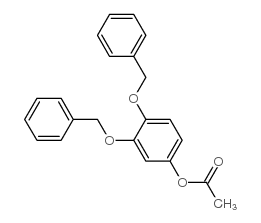 CAS#:27688-85-3
CAS#:27688-85-3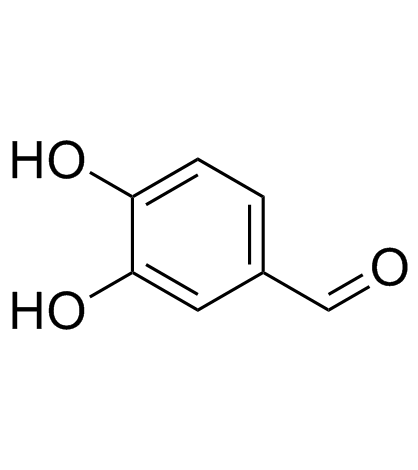 CAS#:139-85-5
CAS#:139-85-5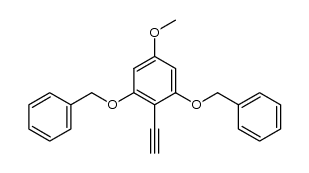 CAS#:627489-10-5
CAS#:627489-10-5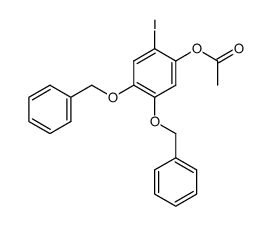 CAS#:627489-15-0
CAS#:627489-15-0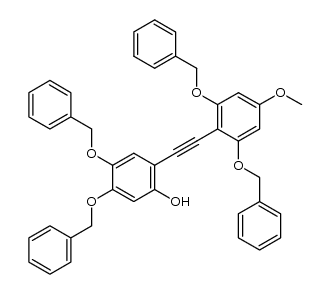 CAS#:344303-12-4
CAS#:344303-12-4
