Lycopene
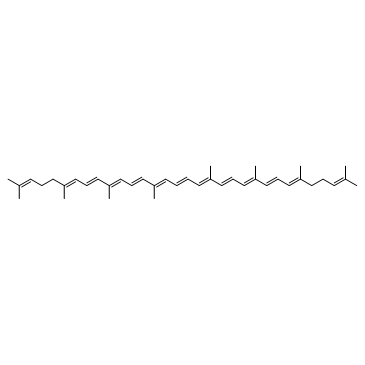
Lycopene structure
|
Common Name | Lycopene | ||
|---|---|---|---|---|
| CAS Number | 502-65-8 | Molecular Weight | 536.873 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 660.9±30.0 °C at 760 mmHg | |
| Molecular Formula | C40H56 | Melting Point | 172-173°C | |
| MSDS | Chinese USA | Flash Point | 350.7±19.4 °C | |
Use of LycopeneLycopene is naturally occurring carotenoids found in tomato, tomato products, and in other red fruits and vegetables; exhibits antioxidant effects. |
| Name | lycopene |
|---|---|
| Synonym | More Synonyms |
| Description | Lycopene is naturally occurring carotenoids found in tomato, tomato products, and in other red fruits and vegetables; exhibits antioxidant effects. |
|---|---|
| Related Catalog | |
| In Vitro | Sufficient uptake of lycopene from the diet is necessary to benefit from its health promoting effects, since humans are unable to synthesise lycopene de novo. Lycopene significantly inhibits prostate and breast cancer cell growth at physiologically relevant concentrations of 1.25 μM and also causes a 30-40 % reduction in inhibitor of kappa B phosphorylation in the cells[1]. Increased intake of lycopene, a major carotenoid in tomatoes, consumed as the all-trans-isomer attenuates alcohol induced apoptosis in 2E1 cells and reduces risk of prostate, lung and digestive cancers. Lycopene plays a role in the protection against photooxidative processes by acting as singlet molecular oxygen and peroxyl radicals scavengers and can interact synergistically with other antioxidants[2]. Lycopene as a carotenoid can react with types of reactive oxygen species (ROS) in three different mechanisms: I) by electron-transfer, II) by hydrogen atom transfer or III) by adduct formation. Lycopene is able to deactivate singlet oxygen mainly by physical quenching[3]. Lycopene decreases ROS production in SK-Hep-1 cells through inhibition of NADPH oxidase, brought about in the PKC pathway[5]. |
| In Vivo | Lycopene is the most predominant carotenoid in human plasma and has a half life of about 2-3 days[2]. Lycopene or processed tomatoes may lead to a reduction of intima-media thickness in vessel walls[3]. Lycopene exerts protective effects against ATZ-induced toxicity in rat adrenal cortex. These effects may be attributed to the antioxidative property of lycopene and its ability to activate the Nrf2/HO-1 pathway[4]. Lycopene improves hepatotoxicity acting as an antioxidant, reduces GSSG and regulates tGSH and CAT levels, reduces oxidative damage[5]. |
| Cell Assay | PC3 cells and MDA-MB-231 cells are treated with (0, 0.5, 1.25, 2.5 and 5 μM) lycopene for 48 h. Cell survival/growth is measured using the colorimetric MTS assay method. MTS-PMS complex (20 μL) is added to each well. The catalytic activity of viable cells results in formazan dye production, which is then quantified. Cells are incubated with the dye for 1 h, followed by absorbance reading at 492 nm on a spectrophotometer[1]. |
| Animal Admin | Rats: Lycopene is dissolved in corn oil. 35 adult male albino rats are randomized into five equal groups: untreated control, vehicle control (receives 0.5 mL corn oil/day), lycopene (10 mg/kg b.w./day), ATZ (dissolved in 0.5 mL corn oil 300 mg/kg b.w./day), and ATZ + lycopene. All treatments are given by oral gavage for 4 weeks[4]. |
| References |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 660.9±30.0 °C at 760 mmHg |
| Melting Point | 172-173°C |
| Molecular Formula | C40H56 |
| Molecular Weight | 536.873 |
| Flash Point | 350.7±19.4 °C |
| Exact Mass | 536.438232 |
| LogP | 15.19 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.531 |
| InChIKey | OAIJSZIZWZSQBC-GYZMGTAESA-N |
| SMILES | CC(C)=CCCC(C)=CC=CC(C)=CC=CC(C)=CC=CC=C(C)C=CC=C(C)C=CC=C(C)CCC=C(C)C |
| Storage condition | −70°C |
| Stability | Heat sensitive - store at -70 C. Combustible. Incompatible with strong oxidizing agents. |
|
SECTION 1: Identification of the substance/mixture and of the company/undertaking Product identifiers Product name: Lycopene REACH No.: A registration number is not available for this substance as the substance or its uses are exempted from registration, the annual tonnage does not require a registration or the registration is envisaged for a later registration deadline.
CAS-No.: 502-65-8 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Laboratory chemicals, Manufacture of substances SECTION 2: Hazards identification Classification of the substance or mixture Not a hazardous substance or mixture according to Regulation (EC) No 1272/2008 Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC. Label elements The product does not need to be labelled in accordance with EC directives or respective national laws. Other hazards - none SECTION 3: Composition/information on ingredients Substances : ψ,ψ-Carotene Synonyms 2,6,10,14,19,23,27,31-Octamethyl-dotriaconta- 2,6,8,10,12,14,16,18,20,22,24,26,30-tridecaene Formula: C40H56 Molecular Weight: 536,87 g/mol CAS-No.: 502-65-8 EC-No.: 207-949-1 No components need to be disclosed according to the applicable regulations. SECTION 4: First aid measures Description of first aid measures If inhaled If breathed in, move person into fresh air. If not breathing, give artificial respiration. In case of skin contact Wash off with soap and plenty of water. In case of eye contact Flush eyes with water as a precaution. If swallowed Never give anything by mouth to an unconscious person. Rinse mouth with water. Most important symptoms and effects, both acute and delayed The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in section 11 Indication of any immediate medical attention and special treatment needed no data available SECTION 5: Firefighting measures Extinguishing media Suitable extinguishing media Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide. Special hazards arising from the substance or mixture Carbon oxides Advice for firefighters Wear self contained breathing apparatus for fire fighting if necessary. Further information no data available SECTION 6: Accidental release measures Personal precautions, protective equipment and emergency procedures Avoid dust formation. Avoid breathing vapours, mist or gas. For personal protection see section 8. Environmental precautions Do not let product enter drains. Methods and materials for containment and cleaning up Sweep up and shovel. Keep in suitable, closed containers for disposal. Reference to other sections For disposal see section 13. SECTION 7: Handling and storage Precautions for safe handling Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire protection. For precautions see section 2.2. Conditions for safe storage, including any incompatibilities Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Recommended storage temperature: -70 °C Specific end use(s) A part from the uses mentioned in section 1.2 no other specific uses are stipulated SECTION 8: Exposure controls/personal protection Control parameters Components with workplace control parameters Exposure controls Appropriate engineering controls General industrial hygiene practice. Personal protective equipment Eye/face protection Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU). Skin protection Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use in accordance with applicable laws and good laboratory practices. Wash and dry hands. The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and the standard EN 374 derived from it. Body Protection Choose body protection in relation to its type, to the concentration and amount of dangerous substances, and to the specific work-place., The type of protective equipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace. Respiratory protection Respiratory protection is not required. Where protection from nuisance levels of dusts are desired, use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and approved under appropriate government standards such as NIOSH (US) or CEN (EU). Control of environmental exposure Do not let product enter drains. SECTION 9: Physical and chemical properties Information on basic physical and chemical properties a) AppearanceForm: solid b) Odourno data available c) Odour Thresholdno data available d) pHno data available e) Melting point/freezingno data available point f) Initial boiling point and no data available boiling range g) Flash pointno data available h) Evapouration rateno data available i) Flammability (solid, gas) no data available j) Upper/lowerno data available flammability or explosive limits k) Vapour pressureno data available l) Vapour densityno data available m) Relative densityno data available n) Water solubilityno data available o) Partition coefficient: n- no data available octanol/water p) Auto-ignitionno data available temperature q) Decompositionno data available temperature r) Viscosityno data available s) Explosive propertiesno data available t) Oxidizing propertiesno data available Other safety information no data available SECTION 10: Stability and reactivity Reactivity no data available Chemical stability Stable under recommended storage conditions. Possibility of hazardous reactions no data available Conditions to avoid no data available Incompatible materials Strong oxidizing agents Hazardous decomposition products Other decomposition products - no data available In the event of fire: see section 5 SECTION 11: Toxicological information Information on toxicological effects Acute toxicity no data available Skin corrosion/irritation no data available Serious eye damage/eye irritation no data available Respiratory or skin sensitisation no data available Germ cell mutagenicity no data available Carcinogenicity IARC:No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC. Reproductive toxicity no data available Specific target organ toxicity - single exposure no data available Specific target organ toxicity - repeated exposure no data available Aspiration hazard no data available Additional Information RTECS: Not available To the best of our knowledge, the chemical, physical, and toxicological properties have not been thoroughly investigated. SECTION 12: Ecological information Toxicity no data available Persistence and degradability no data available Bioaccumulative potential no data available Mobility in soil no data available Results of PBT and vPvB assessment PBT/vPvB assessment not available as chemical safety assessment not required/not conducted Other adverse effects no data available SECTION 13: Disposal considerations Waste treatment methods Product Offer surplus and non-recyclable solutions to a licensed disposal company. Contaminated packaging Dispose of as unused product. SECTION 14: Transport information UN number ADR/RID: -IMDG: -IATA: - UN proper shipping name ADR/RID: Not dangerous goods IMDG: Not dangerous goods IATA:Not dangerous goods Transport hazard class(es) ADR/RID: -IMDG: -IATA: - Packaging group ADR/RID: -IMDG: -IATA: - Environmental hazards ADR/RID: noIMDG Marine pollutant: noIATA: no Special precautions for user no data available SECTION 15 - REGULATORY INFORMATION N/A SECTION 16 - ADDITIONAL INFORMATION N/A |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 3203001990 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 3203001990 |
|---|
|
Chemoprevention of prostate cancer by major dietary phytochemicals.
Anticancer Res. 33(10) , 4163-74, (2013) Prostate cancer continues to be one of the most commonly diagnosed diseases and the second leading cause of cancer-related deaths among men in the United States. Options exist to treat localized disea... |
|
|
Ethylene degreening modulates health promoting phytochemicals in Rio Red grapefruit.
Food Chem. 188 , 77-83, (2015) In the current study, we examined the effects of postharvest degreening and storage on phytochemicals in Rio Red grapefruit. Grapefruits were degreened with 3.5 μl/l of ethylene at 21 °C and 80% relat... |
|
|
An improved UHPLC-UV method for separation and quantification of carotenoids in vegetable crops.
Food Chem. 165 , 475-82, (2014) Carotenoid identification and quantitation is critical for the development of improved nutrition plant varieties. Industrial analysis of carotenoids is typically carried out on multiple crops with pot... |
| lycopen |
| LYCOSOURCE |
| y,y-Carotene |
| Lycopene,Redivivo |
| MFCD00017350 |
| ψ,ψ-Carotene |
| LYCOVIT |
| Lycopin |
| E 160d |
| Lycopene all-trans- |
| Lycopene |
| EINECS 207-949-1 |
| (all-E)-2,6,10,14,19,23,27,31-Octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene |
| (all-trans)-Lycopene |
| y-Carotene |
| (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-Octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaene |
| (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-Octaméthyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridécaène |
| all-trans-Lyc |
| (6E,8E,10E,12E,14E,16E,18E,20E,22E,24E,26E)-2,6,10,14,19,23,27,31-Octamethyl-2,6,8,10,12,14,16,18,20,22,24,26,30-dotriacontatridecaen |
| 4,4-CAROTENE |
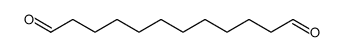 CAS#:38279-34-4
CAS#:38279-34-4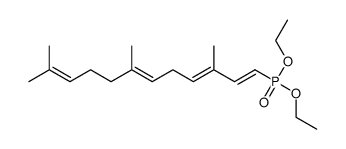 CAS#:1239885-49-4
CAS#:1239885-49-4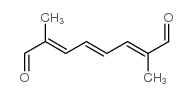 CAS#:5056-17-7
CAS#:5056-17-7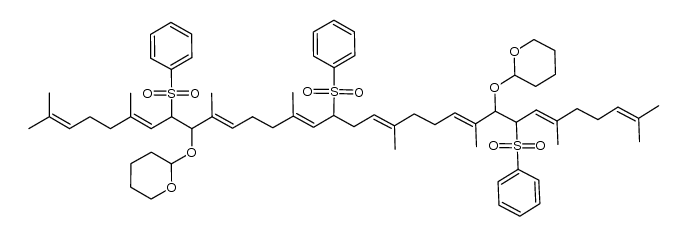 CAS#:1116695-25-0
CAS#:1116695-25-0 CAS#:1116695-27-2
CAS#:1116695-27-2 CAS#:1116695-24-9
CAS#:1116695-24-9 CAS#:359643-76-8
CAS#:359643-76-8 CAS#:1116695-26-1
CAS#:1116695-26-1 CAS#:630426-43-6
CAS#:630426-43-6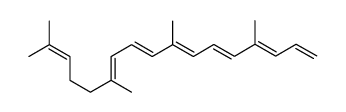 CAS#:1353023-72-9
CAS#:1353023-72-9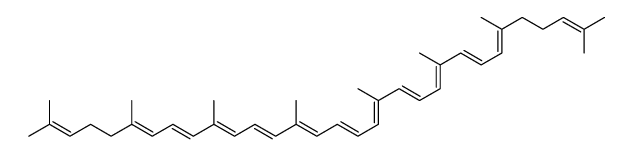 CAS#:4418-71-7
CAS#:4418-71-7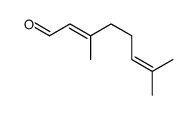 CAS#:141-27-5
CAS#:141-27-5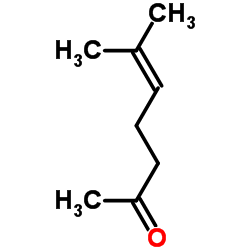 CAS#:110-93-0
CAS#:110-93-0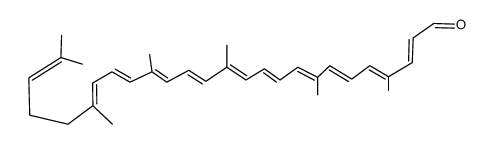 CAS#:22255-36-3
CAS#:22255-36-3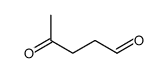 CAS#:626-96-0
CAS#:626-96-0 CAS#:67-64-1
CAS#:67-64-1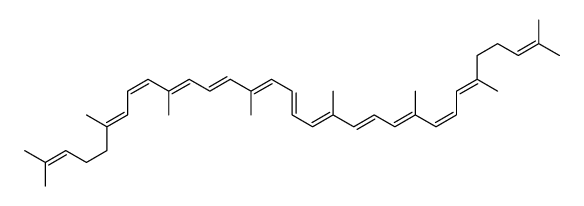 CAS#:13018-46-7
CAS#:13018-46-7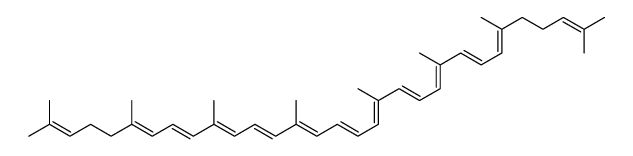 CAS#:64727-64-6
CAS#:64727-64-6 CAS#:101468-86-4
CAS#:101468-86-4
