Selisistat (EX 527)
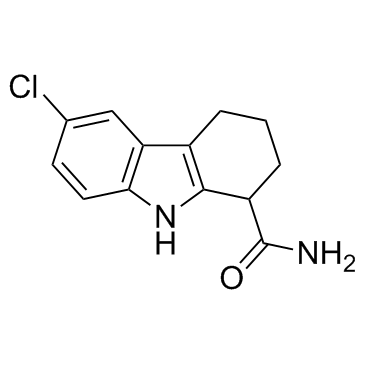
Selisistat (EX 527) structure
|
Common Name | Selisistat (EX 527) | ||
|---|---|---|---|---|
| CAS Number | 49843-98-3 | Molecular Weight | 248.708 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 531.7±38.0 °C at 760 mmHg | |
| Molecular Formula | C13H13ClN2O | Melting Point | 179.0 to 183.0 °C | |
| MSDS | Chinese USA | Flash Point | 275.4±26.8 °C | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of Selisistat (EX 527)Selisistat (EX-527) is a potent and selective SIRT1 inhibitor with IC50 of 98 nM. |
| Name | 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Selisistat (EX-527) is a potent and selective SIRT1 inhibitor with IC50 of 98 nM. |
|---|---|
| Related Catalog | |
| Target |
SIRT1:98 nM (IC50) SIRT2:19.6 μM (IC50) SIRT3:48.7 μM (IC50) |
| In Vitro | Selisistat (EX-527) is an inhibitor of SIRT1 enzymatic activity (IC50, 98 nM), identified in a high-throughput screen using bacterially expressed human SIRT1. Selisistat (EX-527) inhibits SIRT1 in a concentration-dependent manner with an IC50 of 38 nM, in agreement with the activity on bacterially expressed SIRT1. Selisistat (EX-527) has much lower potency against SIRT2 (IC50, 19.6 μM) or SIRT3 (IC50, 48.7 μM) but does not inhibit class I/II HDAC activity at concentrations up to 100 μM[1]. Selisistat (EX-527) exerts an inhibitory effect on SIRT1 activity without affecting SIRT1 expression on both mRNA and protein levels[2]. |
| In Vivo | Selisistat (EX-527) abolishes Resveratrol (RSV)-induced attenuation of microvascular inflammation in ob/ob septic mice. Finally, ob/ob mice in Sepsis+RSV group has significantly increased 7-day survival vs. Sepsis+Vehicle group[3]. |
| Cell Assay | The immortal mouse macrophage cell line RAW264.7 are used. Cells are seeded in 96-well dishes at a density of 3 × 103 cells/cm2 and treated with high glucose at the concentrations of 5.6, 11.1, 25 and 30 mM, alone or with SRT1720 (1 μM) or Selisistat(10 μM) for 24 h. The stock solution of SRT1720 or Selisistat is prepared by dissolving each of them (in powder form) respectively in DMSO yielding a concentration of 100 μM and then stored at -80°C. MTT solution (0.5 mg/mL) is then added to each well and cells are incubated for 4 h at 37°C in a 5% CO2 incubator. Subsequently, the supernatant is removed, the formation of farmazan is solubilized with DMSO and measured at 540 nm with a Bio-Rad Model 680 Plate Reader[2]. |
| Animal Admin | Mice[3]Mice are injected with Resveratrol (RSV) 30mg/kg (4 mL/kg) or equivalent volume of DMSO (Vehicle) (4 mL/kg) intraperitoneally 18 hours pre-sepsis. This dose of RSV in mice is as per documented literature. In one group of mice, RSV pre-treated mice receive Selisistat (10 mg/kg intraperitoneally; 4mL/kg, Vehicle: DMSO) within 10 minutes of cecal ligation and puncture. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 531.7±38.0 °C at 760 mmHg |
| Melting Point | 179.0 to 183.0 °C |
| Molecular Formula | C13H13ClN2O |
| Molecular Weight | 248.708 |
| Flash Point | 275.4±26.8 °C |
| Exact Mass | 248.071640 |
| PSA | 58.88000 |
| LogP | 2.22 |
| Appearance of Characters | white to beige |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.688 |
| Storage condition | Store at +4°C |
| Water Solubility | Soluble in dimethyl sulfoxide, ethanol and dimethyl formamide. |
|
Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression.
Cell Death Dis. 6 , e1827, (2015) Disturbed cell autophagy is found in various cardiovascular disease conditions. Biomechanical stimuli induced by laminar blood flow have important protective actions against the development of various... |
|
|
Resveratrol alleviates the cytotoxicity induced by the radiocontrast agent, ioxitalamate, by reducing the production of reactive oxygen species in HK-2 human renal proximal tubule epithelial cells in vitro.
Int. J. Mol. Med. 37 , 83-91, (2015) Radiocontrast-induced nephropathy (RIN) is one of the leading causes of hospital-acquired acute kidney injury (AKI). The clinical strategies currently available for the prevention of RIN are insuffici... |
|
|
Immunogenicity of the Plasmodium falciparum PfEMP1-VarO Adhesin: Induction of Surface-Reactive and Rosette-Disrupting Antibodies to VarO Infected Erythrocytes.
Stem Cells Dev. 23(20) , 2435-42, (2014) Adhesion of Plasmodium falciparum-infected red blood cells (iRBC) to human erythrocytes (i.e. rosetting) is associated with severe malaria. Rosetting results from interactions between a subset of vari... |
| 6-Chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide,racemic |
| 6-Chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide |
| UNII:L19ECD5014 |
| EX 527 |
| 1H-Carbazole-1-carboxamide, 6-chloro-2,3,4,9-tetrahydro- |
| 6-chloro-1,2,3,4,9-pentahydro-4aH-carbazolecarboxamide |
| SIRT1 Inhibitor III |
| Selisistat |
| 6-Chlor-1,2,3,4-tetrahydrocarbazol-1-carboxamid |
| 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxylic acid amide |
| 6-chloro-2,3,4,9-tetrahydro-1-H-carbazole-1-carboxamide |
| EX-527 |
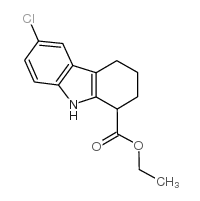 CAS#:49844-36-2
CAS#:49844-36-2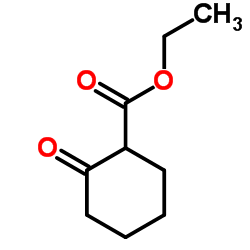 CAS#:1655-07-8
CAS#:1655-07-8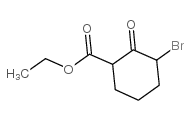 CAS#:30132-23-1
CAS#:30132-23-1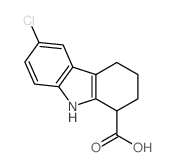 CAS#:50639-66-2
CAS#:50639-66-2