Iretol
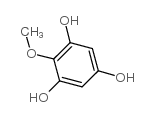
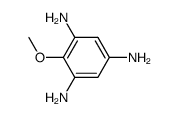
Iretol structure
|
Common Name | Iretol | ||
|---|---|---|---|---|
| CAS Number | 487-71-8 | Molecular Weight | 156.13600 | |
| Density | 1.436g/cm3 | Boiling Point | 323.1ºC at 760 mmHg | |
| Molecular Formula | C7H8O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 149.2ºC | |
Use of IretolIretol (2,4,6-trihydroxyanisole) is a a degradation product of a glucoside obtained from Iris Jorentina. Iretol is an intermediate in the synthesis of natural isoflavones, such as Tectorigenin, Irigenin and Caviunin[1]. |
| Name | 2-methoxybenzene-1,3,5-triol |
|---|---|
| Synonym | More Synonyms |
| Description | Iretol (2,4,6-trihydroxyanisole) is a a degradation product of a glucoside obtained from Iris Jorentina. Iretol is an intermediate in the synthesis of natural isoflavones, such as Tectorigenin, Irigenin and Caviunin[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Zhu-Ping Xiao, et al. 2,4,6-Trihydroxyanisole. Acta Cryst. (2007). E63, o2941. |
| Density | 1.436g/cm3 |
|---|---|
| Boiling Point | 323.1ºC at 760 mmHg |
| Molecular Formula | C7H8O4 |
| Molecular Weight | 156.13600 |
| Flash Point | 149.2ºC |
| Exact Mass | 156.04200 |
| PSA | 69.92000 |
| LogP | 0.81200 |
| Vapour Pressure | 0.000141mmHg at 25°C |
| Index of Refraction | 1.627 |
| HS Code | 2909499000 |
|---|
|
~% 
Iretol CAS#:487-71-8 |
| Literature: Kohner Monatshefte fuer Chemie, 1899 , vol. 20, p. 928 |
|
~% 
Iretol CAS#:487-71-8 |
| Literature: Damschroder; Shriner Journal of the American Chemical Society, 1937 , vol. 59, p. 931 |
|
~% 
Iretol CAS#:487-71-8 |
| Literature: Shibata Yakugaku Zasshi, 1927 , # 543 p. 61 Chem. Zentralbl., 1927 , vol. 98, # II p. 839 |
|
~% 
Iretol CAS#:487-71-8
Detail
|
| Literature: de Laire; Tiemann Chemische Berichte, 1893 , vol. 26, p. 2019 |
| HS Code | 2909499000 |
|---|---|
| Summary | 2909499000. ether-alcohols and their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
| 2,4,6-trihydroxyanisole |
| 1,3,5-trihydroxy-2-methoxybenzene |
| 2,4,6-Trihydroxy-1-methoxy-benzol |
| 1.3.5-Trioxy-2-methoxy-benzol |
| 2-Methoxy-phloroglucin |
| 2-METHOXY-BENZENE-1,3,5-TRIOL |
| 2-Methoxy-phloroglucinol |