Campesterol
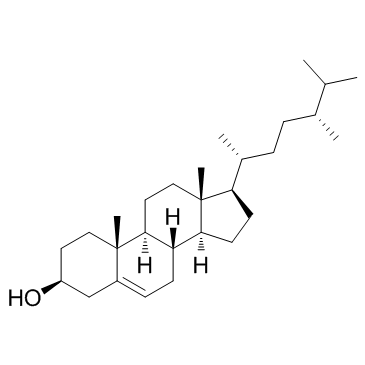
Campesterol structure
|
Common Name | Campesterol | ||
|---|---|---|---|---|
| CAS Number | 474-62-4 | Molecular Weight | 400.680 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 489.5±14.0 °C at 760 mmHg | |
| Molecular Formula | C28H48O | Melting Point | 156-160ºC | |
| MSDS | Chinese USA | Flash Point | 214.3±12.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CampesterolCampesterol is a plant sterol with cholesterol lowering and anticarcinogenic effects. |
| Name | campesterol |
|---|---|
| Synonym | More Synonyms |
| Description | Campesterol is a plant sterol with cholesterol lowering and anticarcinogenic effects. |
|---|---|
| Related Catalog | |
| In Vitro | Campesterol shows a weak cytotoxicity in non-proliferating human umbilical vein endothelial cells (HUVECs). Within the non-cytotoxic concentration range, campesterol significantly inhibits the bFGF-induced proliferation and tube formation of HUVECs in a concentration-dependent manner, while it does not affect the motility of HUVECs. 50 μg/mL of campesterol decreases the cell viability up to about 56% of control(IC50 of over 50 μg/mL)[1]. |
| In Vivo | Campesterol effectively disrupts the bFGF-induced neovascularization in chick chorioallantoic membrane (CAM) in vivo[1]. |
| Cell Assay | The effect of campesterol on the viable cell number was assessed by XTT assay. HUVECs were treated with various concentrations (10, 20, 30, 40 and 50 μg/mL) of campesterol and incubated at 37 °C in a humidified incubator for 24 h, a XTT working solution was added to each well. The cells were incubated at 37 °C for 2 h and the optical density was measured using a microplate reader at 450 nm[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 489.5±14.0 °C at 760 mmHg |
| Melting Point | 156-160ºC |
| Molecular Formula | C28H48O |
| Molecular Weight | 400.680 |
| Flash Point | 214.3±12.4 °C |
| Exact Mass | 400.370514 |
| PSA | 20.23000 |
| LogP | 10.20 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.522 |
| InChIKey | SGNBVLSWZMBQTH-FEGPEKPQSA-N |
| SMILES | CC(C)C(C)CCC(C)C1CCC2C3CC=C4CC(O)CCC4(C)C3CCC12C |
| Storage condition | -20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H315-H319-H332-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful;T: Toxic; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 26-36-45-36/37/39-23 |
| RIDADR | UN 1888 6.1/PG 3 |
|
Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties.
Int. J. Mol. Sci. 16 , 21109-27, (2015) Aerial parts of Veronica species are used in Romanian traditional medicine for the treatment of various conditions like kidney diseases, cough, and catarrh, and are known for their wound-healing prope... |
|
|
Sterol Composition in Infant Formulas and Estimated Intake.
J. Agric. Food Chem. 63 , 7245-51, (2015) Sterol contents in infant formulas (IFs) from the European market were determined, and their intakes by infants between 0 and 6 months were evaluated. Total animal sterols (mg/100 mL) ranged from 1.71... |
|
|
On-line liquid chromatography-gas chromatography: A novel approach for the analysis of phytosterol oxidation products in enriched foods.
J. Chromatogr. A. 1396 , 98-108, (2015) A novel methodology for the automated qualitative and quantitative determination of phytosterol oxidation products in enriched foods via on-line liquid chromatography-gas chromatography (LC-GC) was es... |
| Ergost-5-en-3β-ol, (24R)- |
| (24R)-Ergost-5-en-3b-ol |
| (24R)-5-Ergosten-3β-ol |
| EINECS 207-484-4 |
| (3β,24R)-Ergost-5-en-3-ol |
| (24R)-5-Ergosten-3-β-ol |
| (3b,24R)-Ergost-5-en-3-ol |
| MFCD00010475 |
| (24R)-Ergost-5-en-3β-ol |
| (24R)-5-Ergosten-3b-ol |
| Ergost-5-en-3-ol, (3β)- |
| Ergost-5-en-3β-ol |
| Campesterol |
| (24R)-Ergost-5-en-3-β-ol |
| (3β)-Ergost-5-en-3-ol |
| Ergost-5-en-3-β-ol |
| Ergost-5-en-3-ol, (3β,24R)- |
 CAS#:81477-15-8
CAS#:81477-15-8 CAS#:81477-24-9
CAS#:81477-24-9 CAS#:862499-01-2
CAS#:862499-01-2![(3β)-3-[(Tetrahydro-2H-pyran-2-yl)oxy]-chol-5-en-24-al Structure](https://image.chemsrc.com/caspic/091/66414-44-6.png) CAS#:66414-44-6
CAS#:66414-44-6 CAS#:139403-46-6
CAS#:139403-46-6 CAS#:1900-53-4
CAS#:1900-53-4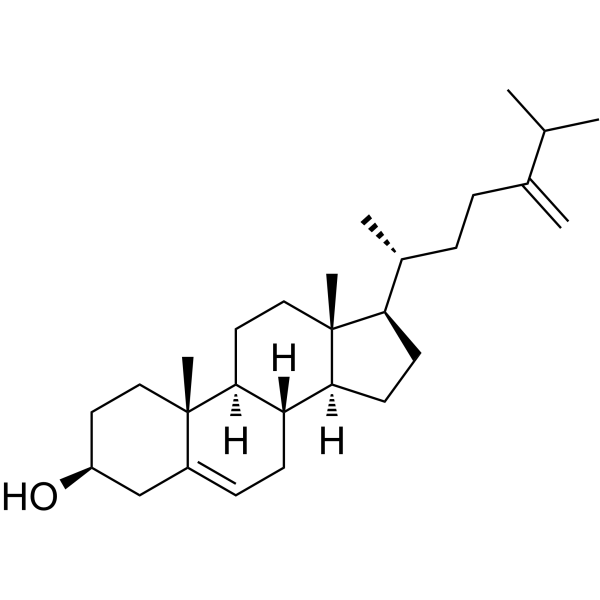 CAS#:474-63-5
CAS#:474-63-5 CAS#:474-60-2
CAS#:474-60-2
