AM1241
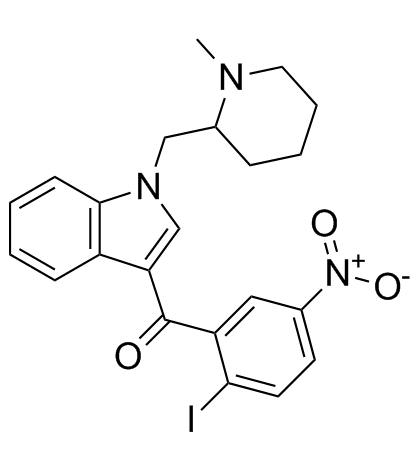
AM1241 structure
|
Common Name | AM1241 | ||
|---|---|---|---|---|
| CAS Number | 444912-48-5 | Molecular Weight | 503.33 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 630.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C22H22IN3O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 335.2±31.5 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of AM1241AM1241 is a potent, typical[2] and selective CB2 receptor agonist, with a Ki of 3.4 nM in a mouse spleen, and the Ki for CB1 receptor in rat brain is 280 nM, 82-fold selectivity for the CB2 receptor in rodent tissue[1]. AM1241 relieves migraine, stroke, and neuropathic pain,also has a potent effect on Parkinson's disease[2]. AM1241 prevents oxidative damage and activates STAT3 through the phosphorylation of Akt and Erk1/2[3]. |
| Name | am-1241 |
|---|---|
| Synonym | More Synonyms |
| Description | AM1241 is a potent, typical[2] and selective CB2 receptor agonist, with a Ki of 3.4 nM in a mouse spleen, and the Ki for CB1 receptor in rat brain is 280 nM, 82-fold selectivity for the CB2 receptor in rodent tissue[1]. AM1241 relieves migraine, stroke, and neuropathic pain,also has a potent effect on Parkinson's disease[2]. AM1241 prevents oxidative damage and activates STAT3 through the phosphorylation of Akt and Erk1/2[3]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 3.4 nM (Mouse spleen CB2 receptor), 280 nM (Rat brain CB1 receptor)[1] |
| In Vitro | AM1241 is a potent and selective CB2 receptor agonist, with a Ki of 3.4 nM in a mouse spleen, and the Ki for CB1 receptor in rat brain is 280 nM, 82-fold selectivity for the CB2 receptor in rodent tissue[1]. AM1241 decreases oxidative stress levels, enhances the production of paracrine growth factors, decreases TGF-β1 and PDGF levels, activates Stat3 via the phosphorylation of Akt and Erk1/2[3]. |
| In Vivo | AM1241 (0.1-3 mg/kg, i.p.) dose-dependently inhibits sensory hypersensitivity in rats. AM1241 inhibits tactile hypersensitivity and thermal hypersensitivity at 1 mg/kg and 3 mg/kg, respectively, in mice lacking the CB1 receptor[1]. AM1241 (0.75, 1.5, 3, 6, 12 mg/kg, i.p.) alleviates MPTP-induced Parkinson's disease and promotes the regeneration of dopaminergic (DA) neurons in PD mice[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 630.7±55.0 °C at 760 mmHg |
| Molecular Formula | C22H22IN3O3 |
| Molecular Weight | 503.33 |
| Flash Point | 335.2±31.5 °C |
| PSA | 71.06000 |
| LogP | 3.41 |
| Appearance of Characters | yellow |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.693 |
| Storage condition | -20℃ |
| Water Solubility | DMSO: ~18mg/mL at 60°C |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H319-H334-H335 |
| Precautionary Statements | P261-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 36/37/38-42/43 |
| Safety Phrases | 22-26-36/37-45 |
| RIDADR | NONH for all modes of transport |
|
Self-medication of a cannabinoid CB2 agonist in an animal model of neuropathic pain.
Pain 152 , 1976-87, (2011) Drug self-administration methods were used to test the hypothesis that rats would self-medicate with a cannabinoid CB(2) agonist to attenuate a neuropathic pain state. Self-medication of the CB(2) ago... |
|
|
Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS.
Proc. Natl. Acad. Sci. U. S. A. 100 , 10529-10533, (2003) We designed AM1241, a selective CB2 cannabinoid receptor agonist, and used it to test the hypothesis that CB2 receptor activation would reverse the sensory hypersensitivity observed in neuropathic pai... |
|
|
Central and peripheral sites of action for CB₂ receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats.
Br. J. Pharmacol. 162 , 428-40, (2011) Cannabinoid CB₂ receptor activation by selective agonists has been shown to produce analgesic effects in preclinical models of inflammatory and neuropathic pain. However, mechanisms underlying CB₂-med... |
| (2-iodo-5-nitrophenyl){1-[(1-methylpiperidin-2-yl)methyl]-1H-indol-3-yl}methanone |
| Dacinostat |
| LAQ-824 |
| (2-Iodo-5-nitrophenyl){1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl}methanone |
| (E)-N-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide |
| UNII-V10P524501 |
| (2-Iodo-5-nitrophenyl)(1-((1-methylpiperidin-2-yl)methyl)-1H-Indol-3-yl)methanone |
| 2-Propenamide, N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-, (2E)- |
| Methanone, (2-iodo-5-nitrophenyl)[1-[(1-methyl-2-piperidinyl)methyl]-1H-indol-3-yl]- |
| (R,S)-3-(2-Iodo-5-nitrobenzoyl)-1-(1-methyl-2-piperidinylmethyl)-1H-indole |
| (2E)-N-Hydroxy-3-[4-({(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino}methyl)phenyl]acrylamide |
| (2E)-N-hydroxy-3-[4-({(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enamide |
| NVP-LAQ824 |
| UNII:DLM851L3RD |
| S1095_Selleck |
| AM1241 |