camp sodium salt
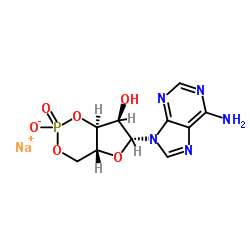
camp sodium salt structure
|
Common Name | camp sodium salt | ||
|---|---|---|---|---|
| CAS Number | 37839-81-9 | Molecular Weight | 351.19 | |
| Density | N/A | Boiling Point | 701.5ºC at 760 mmHg | |
| Molecular Formula | C10H11N5NaO6P | Melting Point | 219 - 220ºC | |
| MSDS | N/A | Flash Point | 378ºC | |
Use of camp sodium saltCyclic AMP (Cyclic adenosine monophosphate) sodium, adenosine triphosphate derivative, is an intracellular signaling molecule responsible for directing cellular responses to extracellular signals. Cyclic AMP sodium is an important second messenger in many biological processes[1][2][3]. |
| Name | 3',5'-cAMP Na Hydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Cyclic AMP (Cyclic adenosine monophosphate) sodium, adenosine triphosphate derivative, is an intracellular signaling molecule responsible for directing cellular responses to extracellular signals. Cyclic AMP sodium is an important second messenger in many biological processes[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite Microbial Metabolite |
| In Vitro | Cyclic AMP (Cyclic adenosine monophosphate) sodium modulates mediator generation. Cyclic AMP sodium suppresses the expression of pro-inflammatory cytokines, including TNF-α and IL-12, and enhances the production of the anti-inflammatory cytokine IL-10. |
| References |
[1]. G M Fimia, et al. Cyclic AMP signaling. J Cell Sci. 2001 Jun;114(Pt 11):1971-2. |
| Boiling Point | 701.5ºC at 760 mmHg |
|---|---|
| Melting Point | 219 - 220ºC |
| Molecular Formula | C10H11N5NaO6P |
| Molecular Weight | 351.19 |
| Flash Point | 378ºC |
| Exact Mass | 351.034454 |
| PSA | 167.48000 |
| LogP | 0.20200 |
| InChIKey | QJOMQLIYJMMGDA-MCDZGGTQSA-N |
| SMILES | Nc1ncnc2c1ncn2C1OC2COP(=O)(O)OC2C1O.[Na] |
| Storage condition | −20°C |
| Water Solubility | H2O: 50 mg/mL | Soluble in water (50 mg/ml). |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 0 | |
|---|---|
| DownStream 1 | |
|
H4 histamine receptors inhibit steroidogenesis and proliferation in Leydig cells.
J. Endocrinol. 223(3) , 241-53, (2014) The histamine H4 receptor (HRH4), discovered only 13 years ago, is considered a promising drug target for allergy, inflammation, autoimmune disorders and cancer, as reflected by a steadily growing num... |
|
|
HBCDD-induced sustained reduction in mitochondrial membrane potential, ATP and steroidogenesis in peripubertal rat Leydig cells.
Toxicol. Appl. Pharmacol. 282(1) , 20-9, (2015) Hexabromocyclododecane (HBCDD), a brominated flame retardant added to various consumer products, is a ubiquitous environmental contaminant. We have previously shown that 6-hour exposure to HBCDD distu... |
|
|
Activation of cAMP signaling attenuates impaired hepatic glucose disposal in aged male p21-activated protein kinase-1 knockout mice.
Endocrinology 155(6) , 2122-32, (2014) p21-activated protein kinase-1 (Pak1) plays a role in insulin secretion and glucagon-like peptide-1 (GLP-1) production. Pak1(-/-) mice were found to carry a defect in ip pyruvate tolerance test (IPPTT... |
| 4H-Furo[3,2-d]-1,3,2-dioxaphosphorin-7-ol, 6-(6-amino-9H-purin-9-yl)tetrahydro-2-hydroxy-, 2-oxide, sodium salt, (4aR,6R,7R,7aS)- (1:1) |
| Adenosine 3',5'-cyclic monophosphate sodium salt monohydrate |
| 3’,5‘-Cyclic AMP sodium salt |
| Adenosine 3',5'-Cyclic Monophosphate Sodium Salt Hydrate |
| Sodium (4aR,6R,7R,7aS)-6-(6-amino-9H-purin-9-yl)-7-hydroxytetrahydro-4H-furo[3,2-d][1,3,2]dioxaphosphinin-2-olate 2-oxide |
| 3',5'-Cyclic AMP Sodium Salt Hydrate |
| Adenosine 3’,5‘-cyclic monophosphate sodium salt monohydrate |
| MFCD00069736 |
| CAMP SODIUM SALT |
 CAS#:61-19-8
CAS#:61-19-8
