4-hydroxyisobenzofuran-1,3-dione

4-hydroxyisobenzofuran-1,3-dione structure
|
Common Name | 4-hydroxyisobenzofuran-1,3-dione | ||
|---|---|---|---|---|
| CAS Number | 37418-88-5 | Molecular Weight | 164.12 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 365.4±25.0 °C at 760 mmHg | |
| Molecular Formula | C8H4O4 | Melting Point | 199-202 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 160.2±16.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-hydroxyisobenzofuran-1,3-dione4-Hydroxyisobenzofuran-1,3-dione is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 3-Hydroxyphthalic anhydride |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Hydroxyisobenzofuran-1,3-dione is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 365.4±25.0 °C at 760 mmHg |
| Melting Point | 199-202 °C(lit.) |
| Molecular Formula | C8H4O4 |
| Molecular Weight | 164.12 |
| Flash Point | 160.2±16.7 °C |
| Exact Mass | 164.010956 |
| PSA | 63.60000 |
| LogP | 0.86 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.666 |
| InChIKey | CCTOEAMRIIXGDJ-UHFFFAOYSA-N |
| SMILES | O=C1OC(=O)c2c(O)cccc21 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TI3300000 |
| HS Code | 2932999099 |
|
~70% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Board of Trustees of Michigan State University Patent: US2005/267307 A1, 2005 ; Location in patent: Page/Page column 4 ; |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Pratt; Perkins Journal of the American Chemical Society, 1918 , vol. 40, p. 230 |
|
~90% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Nasman Synthesis, 1985 , vol. NO. 8, p. 788 - 789 |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Bentley; Robinson; Weizmann Journal of the Chemical Society, 1907 , vol. 91, p. 109 |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Bentley; Robinson; Weizmann Journal of the Chemical Society, 1907 , vol. 91, p. 109 |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Bentley; Robinson; Weizmann Journal of the Chemical Society, 1907 , vol. 91, p. 109 |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Bentley; Robinson; Weizmann Journal of the Chemical Society, 1907 , vol. 91, p. 109 |
|
~% 
4-hydroxyisoben... CAS#:37418-88-5 |
| Literature: Petfield; Amstutz Journal of Organic Chemistry, 1954 , vol. 19, p. 1944 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The antiviral activity of naturally occurring proteins and their peptide fragments after chemical modification.
Antiviral Res. 59(1) , 23-33, (2003) Chemical modification of the proteins bovine serum albumin, alpha-lactalbumin, beta-lactoglobulin and chicken lysozyme by 3-hydroxyphthalic anhydride (3-HP) yielded compounds which exerted antiviral a... |
|
|
[Antiviral activity of 3-hydroxyphthalic anhydride-modified ovalbumin against herpes simplex virus 2 in vitro].
Nan Fang Yi Ke Da Xue Xue Bao 31(7) , 1175-8, (2011) To investigate the antiviral activity of 3-hydroxyphthalic anhydride-modified ovalbumin (HP-OVA) against herpes simplex virus 2 (HSV-2) in vitro.By chemical modification, ovalbumin (OVA) was treated w... |
|
|
3-hydroxyphthalic anhydride-modified chicken ovalbumin exhibits potent and broad anti-HIV-1 activity: a potential microbicide for preventing sexual transmission of HIV-1.
Antimicrob. Agents Chemother. 54(5) , 1700-11, (2010) Heterosexual transmission is the primary route by which women acquire human immunodeficiency virus (HIV)/AIDS. Thus, development of woman-controlled topical microbicides for prevention of sexual trans... |
| MFCD00011557 |
| 3-Hydroxyphthalic anhydride |
| 4-Hydroxyisobenzofuran-1,3-dione |
| 4-Hydroxy-2-benzofuran-1,3-dione |
| 1,3-Isobenzofurandione, 4-hydroxy- |
| 4-Hydroxy-isobenzofuran-1,3-dione |
![1-(2,2-dimethylpropanoyloxy-3,5)-dioxo-exo-10-oxatricyclo[5.2.1.02.6]dec-8-ene structure](https://image.chemsrc.com/caspic/344/135414-50-5.png)
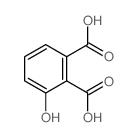

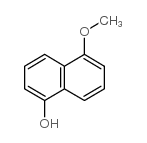
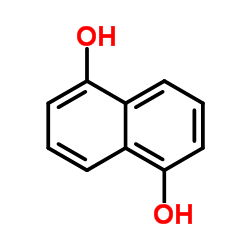
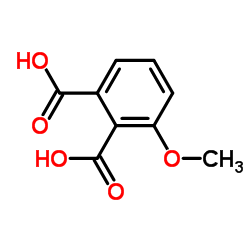


 CAS#:102673-72-3
CAS#:102673-72-3 CAS#:14963-96-3
CAS#:14963-96-3 CAS#:129-43-1
CAS#:129-43-1 CAS#:101976-15-2
CAS#:101976-15-2 CAS#:36669-02-0
CAS#:36669-02-0 CAS#:2961-04-8
CAS#:2961-04-8 CAS#:70767-96-3
CAS#:70767-96-3 CAS#:7369-27-9
CAS#:7369-27-9 CAS#:74272-75-6
CAS#:74272-75-6