Adenosine dialdehyde
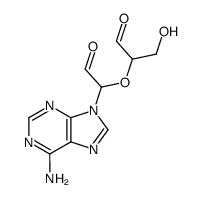
Adenosine dialdehyde structure
|
Common Name | Adenosine dialdehyde | ||
|---|---|---|---|---|
| CAS Number | 34240-05-6 | Molecular Weight | 265.22500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C10H11N5O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of Adenosine dialdehydeAdenosine Dialdehyde is a purine nucleoside analogue and is an irreversible inhibitor of S-adenosylhomocysteine hydrolase (SAH) (IC50=40 nM). Adenosine Dialdehyde exhibits potent anti-tumor activity in vivo and can be used for the cancer research[1][2]. |
| Name | Adenosine, periodate oxidized |
|---|---|
| Synonym | More Synonyms |
| Description | Adenosine Dialdehyde is a purine nucleoside analogue and is an irreversible inhibitor of S-adenosylhomocysteine hydrolase (SAH) (IC50=40 nM). Adenosine Dialdehyde exhibits potent anti-tumor activity in vivo and can be used for the cancer research[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Adenosine dialdehyde suppresses MNB cell replication in tissue culture with concentra tions of 1.5 μM with producing 50% inhibition[1]. |
| In Vivo | Adenosine dialdehyde (subcutaneous injection; 1.5-2.5 mg/kg; infused over a 7-day period ( minipump infusion)) significantly increases the mean life span of tumor bearing mice from 20.9 days in diluent treated controls to 35.3 days in AD treated animals[2]. Adenosine dialdehyde (subcutaneous injection; 1.5-2.5 mg/kg; two 7-day periods interspersed by a 7-day drug free interval( minipump infusion))increases mean life span 80% in diluent treated controls (controls, 21.3 days; AD treated 38.4 days) in mice[2]. Adenosine dialdehyde (subcutaneous injection; 2-3 mg/kg; infused over a 7-day period ( minipump infusion)) does not exhibit any hematopoietic toxicity in mice, and it can significantly suppress murine neuroblastoma tumor growth with little systemic toxicity[2]. Animal Model: Adult male A/J mice, weighing 20 to 25 g with MNB cells[2] Dosage: 1.5-2.5 mg/kg Administration: Subcutaneous injection; 1.5-2.5 mg/kg; two 7-day periods interspersed by a 7-day drug free interval (minipump infusion) Result: Significantly suppressed murine neuroblastoma tumor growth. Prolongs the life span of tumor bearing mice. Did not suppress hematopoiesis when administered by steady state infusion[2]. |
| References |
| Molecular Formula | C10H11N5O4 |
|---|---|
| Molecular Weight | 265.22500 |
| Exact Mass | 265.08100 |
| PSA | 133.22000 |
| Storage condition | -20℃ |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Elasticity assessment of electrospun nanofibrous vascular grafts: a comparison with femoral ovine arteries.
Mater. Sci. Eng. C. Mater. Biol. Appl. 45 , 446-54, (2014) Development of successful small-diameter vascular grafts constitutes a real challenge to biomaterial engineering. In most cases these grafts fail in-vivo due to the presence of a mechanical mismatch b... |
|
|
Efflux of glutathione and glutathione complexes from human erythrocytes in response to vanadate.
Blood Cells Mol. Dis. 50(1) , 1-7, (2013) The main objective of the present study was to investigate if vanadate is extruded from the cells in a glutathione dependent manner resulting in the appearance of extracellular glutathione and complex... |
|
|
Involvement of Src and the actin cytoskeleton in the antitumorigenic action of adenosine dialdehyde.
Biochem. Pharmacol. 85(8) , 1042-56, (2013) Transmethylation is an important reaction that transfers a methyl group in S-adenosylmethionine (SAM) to substrates such as DNA, RNA, and proteins. It is known that transmethylation plays critical rol... |
| ADENOSINE, PERIODATE OXIDIZED |
| α-adenosine 2',3'-dialdehyde |
| ADENOSINE, PERIODATE OXIDISED |
| Adenosine Dialdehyde |