4-nitrocatechol
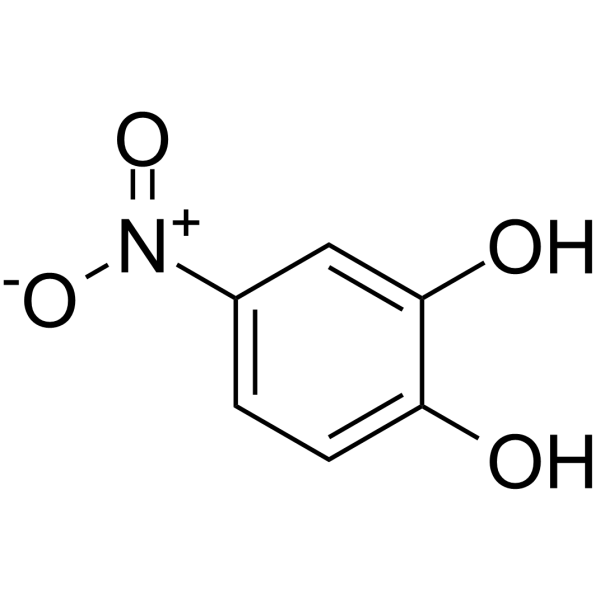
4-nitrocatechol structure
|
Common Name | 4-nitrocatechol | ||
|---|---|---|---|---|
| CAS Number | 3316-09-4 | Molecular Weight | 155.108 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 358.2±32.0 °C at 760 mmHg | |
| Molecular Formula | C6H5NO4 | Melting Point | 173-177 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 168.8±13.6 °C | |
Use of 4-nitrocatechol4-Nitrocatechol is a potent lipoxygenase inhibitor[1]. |
| Name | 4-nitrocatechol |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Nitrocatechol is a potent lipoxygenase inhibitor[1]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite Microbial Metabolite |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 358.2±32.0 °C at 760 mmHg |
| Melting Point | 173-177 °C(lit.) |
| Molecular Formula | C6H5NO4 |
| Molecular Weight | 155.108 |
| Flash Point | 168.8±13.6 °C |
| Exact Mass | 155.021851 |
| PSA | 86.28000 |
| LogP | 1.68 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26 |
| RIDADR | 2811 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2908999090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2908999090 |
|---|---|
| Summary | 2908999090 halogenated, sulphonated, nitrated or nitrosated derivatives of phenols or phenol-alcohols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Effect of commercially available green and black tea beverages on drug-metabolizing enzymes and oxidative stress in Wistar rats.
Food Chem. Toxicol. 70 , 120-7, (2014) The effect of commercially available green tea (GT) and black tea (BT) drinks on drug metabolizing enzymes (DME) and oxidative stress in rats was investigated. Male Wistar rats were fed a laboratory c... |
|
|
Molecular characterization of the boron adducts of the proteasome inhibitor bortezomib with epigallocatechin-3-gallate and related polyphenols.
Org. Biomol. Chem. 13(13) , 3887-99, (2015) The green tea polyphenol epigallocatechin-3-gallate (EGCG) was reported to effectively antagonize the ability of Bortezomib (BZM) to induce apoptosis in cancer cells. This interaction was attributed t... |
|
|
Phase II metabolism in human skin: skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation.
Drug Metab. Dispos. 43(1) , 126-39, (2014) Although skin is the largest organ of the human body, cutaneous drug metabolism is often overlooked, and existing experimental models are insufficiently validated. This proof-of-concept study investig... |
| EINECS 222-009-0 |
| 1,2-Benzenediol, 4-nitro- |
| 1,2-dihydroxy-4-nitrobenzene |
| [14C]-4-Nitrocatechol |
| NITROCATECHOL (MIX OF ISOMERS) |
| 4-nitrocathecol |
| 3,4-dihydroxynitrobenzene |
| 4-Nitro-1,2-benzenediol |
| 4-nitrocatechol |
| 4-Nitrobenzol-1,2-diol |
| MFCD00007242 |
| 4-Nitropyrocatechol |
| 4-nitrobenzene-1,2-diol |
 CAS#:619-08-9
CAS#:619-08-9 CAS#:97-51-8
CAS#:97-51-8 CAS#:2460-58-4
CAS#:2460-58-4 CAS#:2620-44-2
CAS#:2620-44-2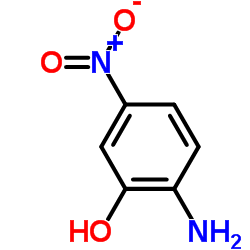 CAS#:121-88-0
CAS#:121-88-0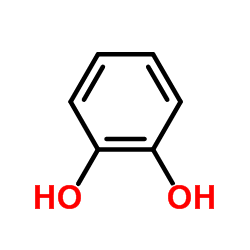 CAS#:120-80-9
CAS#:120-80-9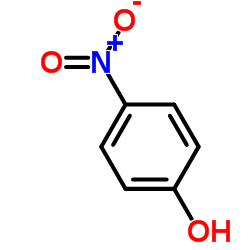 CAS#:100-02-7
CAS#:100-02-7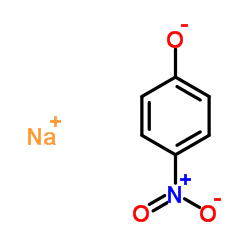 CAS#:824-78-2
CAS#:824-78-2![7-nitro-benzo[1,3]dioxole-5-carboxylic acid Structure](https://image.chemsrc.com/caspic/243/7112-72-3.png) CAS#:7112-72-3
CAS#:7112-72-3 CAS#:1450-76-6
CAS#:1450-76-6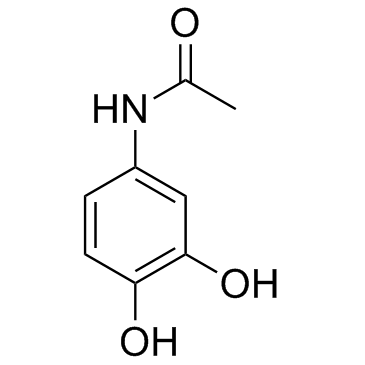 CAS#:37519-14-5
CAS#:37519-14-5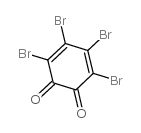 CAS#:2435-54-3
CAS#:2435-54-3 CAS#:488-47-1
CAS#:488-47-1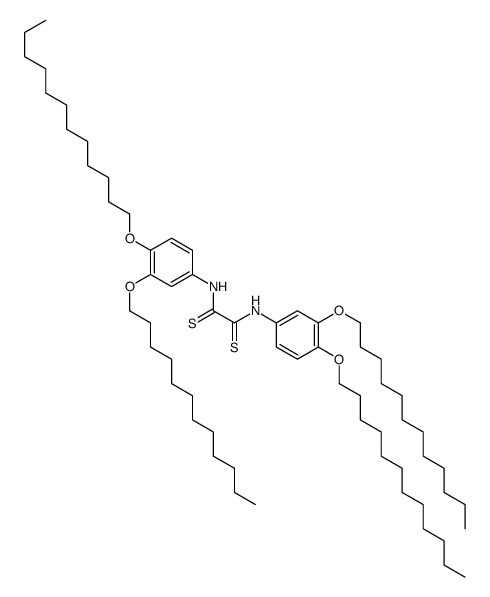 CAS#:189934-25-6
CAS#:189934-25-6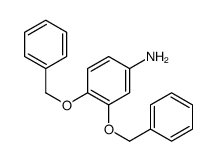 CAS#:18002-44-3
CAS#:18002-44-3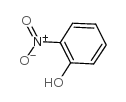 CAS#:88-75-5
CAS#:88-75-5 CAS#:78288-94-5
CAS#:78288-94-5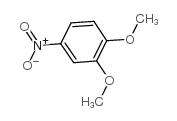 CAS#:709-09-1
CAS#:709-09-1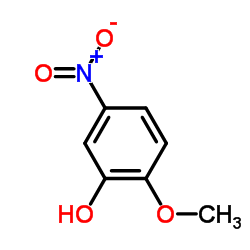 CAS#:636-93-1
CAS#:636-93-1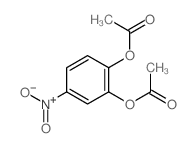 CAS#:36383-33-2
CAS#:36383-33-2
