glycyl-prolyl-glutamic acid
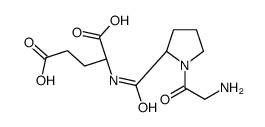
glycyl-prolyl-glutamic acid structure
|
Common Name | glycyl-prolyl-glutamic acid | ||
|---|---|---|---|---|
| CAS Number | 32302-76-4 | Molecular Weight | 301.29600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C12H19N3O6 | Melting Point | N/A | |
| MSDS | Chinese | Flash Point | N/A | |
Use of glycyl-prolyl-glutamic acidGly-Pro-Glu is a neuroactive peptide with a potent action on acetylcholine release. Gly-Pro-Glu is the N-terminal tripeptide of insulin-like growth factor-I. Gly-Pro-Glu inhibits glutamate binds to N-methyl-D-aspartate (NMDA) receptor with an IC50 value of 14.7 μM. Gly-Pro-Glu can be used for the research of neuroprotection [1][2]. |
| Name | (2S)-2-[[(2S)-1-(2-aminoacetyl)pyrrolidine-2-carbonyl]amino]pentanedioic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Gly-Pro-Glu is a neuroactive peptide with a potent action on acetylcholine release. Gly-Pro-Glu is the N-terminal tripeptide of insulin-like growth factor-I. Gly-Pro-Glu inhibits glutamate binds to N-methyl-D-aspartate (NMDA) receptor with an IC50 value of 14.7 μM. Gly-Pro-Glu can be used for the research of neuroprotection [1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 14.7 μM (glutamate binds to NMDA receptor)[1] |
| In Vitro | Gly-Pro-Glu (0-100 μM) potentiates the potassium evoked release of both acetylcholine and dopamine, increases K+ evoked acetylcholine release even at concentrations of 0.1 nM and significantly enhances evoked dopamine release[1]. Gly-Pro-Glu (1-1000 μM) shows an inhibition of L-[3H]glutamate binding with an IC50 value of 14.7 μM[1]. |
| In Vivo | Gly-Pro-Glu (300 mg; i.p. once per day; on day 0, 6 and 12) shows an in vivo effect protecting the temporal cortical somatostatinergic system from Abeta insult.[2]. Animal Model: Ovariectomized rats with Abeta25-35 injection[2] Dosage: 300 mg Administration: Intraperitoneal injection; 300 mg per day; on day 0, 6 and 12 Result: Recovered Abeta25-35-induced the reduction of somatostatin (SRIF) content and SRIF receptor density, and reduced the inhibitory effect of SRIF on adenylyl cyclase activity. |
| Molecular Formula | C12H19N3O6 |
|---|---|
| Molecular Weight | 301.29600 |
| Exact Mass | 301.12700 |
| PSA | 153.52000 |
| InChIKey | JJGBXTYGTKWGAT-YUMQZZPRSA-N |
| SMILES | NCC(=O)N1CCCC1C(=O)NC(CCC(=O)O)C(=O)O |
| Storage condition | Store at 0°C |
| Water Solubility | H2O: >5mg/mL |
| RIDADR | NONH for all modes of transport |
|---|
|
Ethyl acetate extract from Glycosmis parva leaf induces apoptosis and cell-cycle arrest by decreasing expression of COX-2 and altering BCL-2 family gene expression in human colorectal cancer HT-29 cells.
Pharm. Biol. 53(4) , 540-7, (2015) Glycosmis parva Craib (Rutaceae) is reported to have cytotoxic and anti-inflammatory activities by decreasing COX-2 expression.To investigate the effect of G. parva on human colorectal cancer cells ex... |
|
|
Analogues of the neuroprotective tripeptide Gly-Pro-Glu (GPE): synthesis and structure-activity relationships.
Bioorg. Med. Chem. Lett. 15 , 2279-83, (2005) A series of GPE analogues, including modifications at the Pro and/or Glu residues, was prepared and evaluated for their NMDA binding and neuroprotective effects. Main results suggest that the pyrrolid... |
|
|
Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages.
PLoS Pathog. 7 , e1002047, (2011) A remarkable aspect of the interaction of Cryptococcus neoformans with mammalian hosts is a consistent increase in capsule volume. Given that many aspects of the interaction of C. neoformans with macr... |
| Gly-pro-glu |
| GPE Glycyl-prolyl-glutamic acid |
| Glycyl-prolyl-glutamic acid |
| L-Glutamic acid,N-(1-glycyl-L-prolyl) |