| Structure | Name/CAS No. | Articles |
|---|---|---|
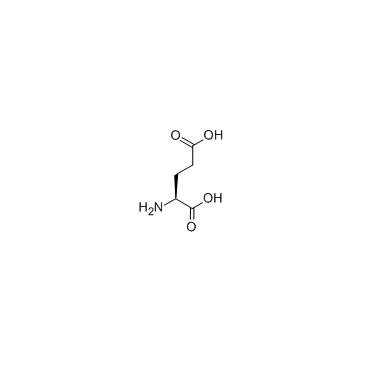 |
L-glutamic acid
CAS:56-86-0 |
|
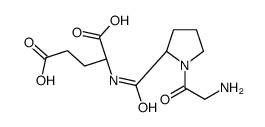 |
glycyl-prolyl-glutamic acid
CAS:32302-76-4 |
|
 |
D-AP5(mM/ml)
CAS:79055-68-8 |