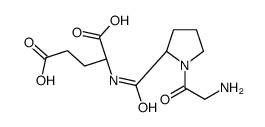
32302-76-4
| Name | (2S)-2-[[(2S)-1-(2-aminoacetyl)pyrrolidine-2-carbonyl]amino]pentanedioic acid |
|---|---|
| Synonyms |
Gly-pro-glu
GPE Glycyl-prolyl-glutamic acid Glycyl-prolyl-glutamic acid L-Glutamic acid,N-(1-glycyl-L-prolyl) |
| Description | Gly-Pro-Glu is a neuroactive peptide with a potent action on acetylcholine release. Gly-Pro-Glu is the N-terminal tripeptide of insulin-like growth factor-I. Gly-Pro-Glu inhibits glutamate binds to N-methyl-D-aspartate (NMDA) receptor with an IC50 value of 14.7 μM. Gly-Pro-Glu can be used for the research of neuroprotection [1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 14.7 μM (glutamate binds to NMDA receptor)[1] |
| In Vitro | Gly-Pro-Glu (0-100 μM) potentiates the potassium evoked release of both acetylcholine and dopamine, increases K+ evoked acetylcholine release even at concentrations of 0.1 nM and significantly enhances evoked dopamine release[1]. Gly-Pro-Glu (1-1000 μM) shows an inhibition of L-[3H]glutamate binding with an IC50 value of 14.7 μM[1]. |
| In Vivo | Gly-Pro-Glu (300 mg; i.p. once per day; on day 0, 6 and 12) shows an in vivo effect protecting the temporal cortical somatostatinergic system from Abeta insult.[2]. Animal Model: Ovariectomized rats with Abeta25-35 injection[2] Dosage: 300 mg Administration: Intraperitoneal injection; 300 mg per day; on day 0, 6 and 12 Result: Recovered Abeta25-35-induced the reduction of somatostatin (SRIF) content and SRIF receptor density, and reduced the inhibitory effect of SRIF on adenylyl cyclase activity. |
| Molecular Formula | C12H19N3O6 |
|---|---|
| Molecular Weight | 301.29600 |
| Exact Mass | 301.12700 |
| PSA | 153.52000 |
| Storage condition | Store at 0°C |
| Water Solubility | H2O: >5mg/mL |
| RIDADR | NONH for all modes of transport |
|---|