H-Phe-ol
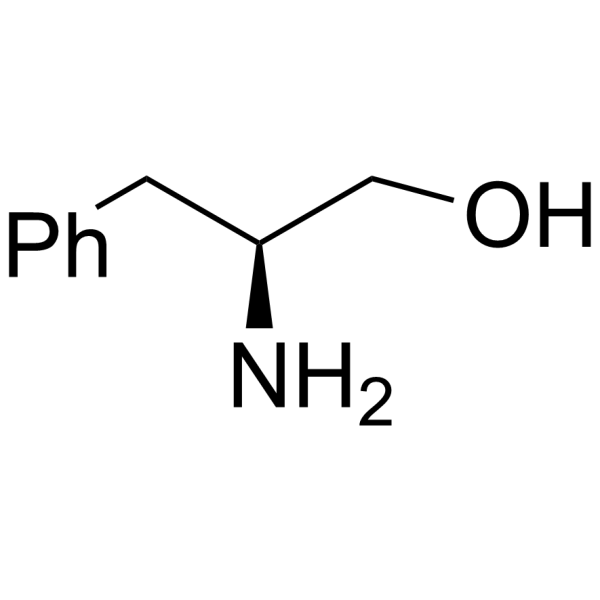
H-Phe-ol structure
|
Common Name | H-Phe-ol | ||
|---|---|---|---|---|
| CAS Number | 3182-95-4 | Molecular Weight | 151.206 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 303.8±22.0 °C at 760 mmHg | |
| Molecular Formula | C9H13NO | Melting Point | 92-94ºC | |
| MSDS | Chinese USA | Flash Point | 137.5±22.3 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
Use of H-Phe-olL-(-)-Phenylalaninol is an alanine derivative[1]. |
| Name | (2S)-2-amino-3-phenylpropan-1-ol |
|---|---|
| Synonym | More Synonyms |
| Description | L-(-)-Phenylalaninol is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 303.8±22.0 °C at 760 mmHg |
| Melting Point | 92-94ºC |
| Molecular Formula | C9H13NO |
| Molecular Weight | 151.206 |
| Flash Point | 137.5±22.3 °C |
| Exact Mass | 151.099716 |
| PSA | 46.25000 |
| LogP | 0.77 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.561 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C: Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S24/25 |
| RIDADR | UN 3259 8/PG 3 |
| WGK Germany | 3 |
| RTECS | UA6900000 |
| Hazard Class | 8.0 |
| HS Code | 29221980 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Straightforward methodology for the enantioselective synthesis of benzo[a]- and indolo[2,3-a]quinolizidines.
J. Org. Chem. 72(14) , 5193-201, (2007) An enantioselective two-step route to substituted benzo[a]- and indolo[2,3-a]quinolizidines has been developed. It consists of (i) a stereoselective cyclocondensation of a racemic or prochiral delta-o... |
|
|
Direct high-performance liquid chromatographic separation of the enantiomers of an aromatic amine and four aminoalcohols using polysaccharide chiral stationary phases and acidic additive.
Chirality 19(8) , 647-53, (2007) The HPLC enantiomeric separation of N-benzyl-alpha-methyl-benzylamine, phenylalaninol, tryptophanol, 2 (diphenylhydroxymethyl)pyrrolidine, and isoproterenol was accomplished in the normal-phase mode u... |
|
|
Paracelsin; characterization by NMR spectroscopy and circular dichroism, and hemolytic properties of a peptaibol antibiotic from the cellulolytically active mold Trichoderma reesei. Part B.
Experientia 40 , 1189, (1984) Paracelsin, a hemolytic and membrane active polypeptide antibiotic of the peptaibol class which is excreted by the mold Trichoderma reesei, was obtained by a simplified and rapid isolation procedure u... |
| Ethanol, 2- (phenylamino)- |
| β-Anilinoethanol |
| HPH |
| (S)-(-)-2-amino-3-phenylpropan-1-ol |
| (S)-2-Amino-3-phenyl-1-propanol |
| (S)-2-Amino-3-phenylpropan-1-ol |
| (S)-2-amino-2-phenylpropan-1-ol |
| (S)-2-Amino-3-phenyl-propan-1-ol |
| N-(β-hydroxy)ethylaniline |
| Aniline, N-(β-hydroxyethyl)- |
| Ethanol, 2-(phenylamino)- |
| L(-)-2-Amino-3-phenyl-1-propanol |
| 2-(phenylamino)ethanol |
| EINECS 221-674-4 |
| H-Phenylalaninol |
| (S)-(-)-2-Amino-3-phenyl-1-propanol |
| (S)-(-)-phenylalaninol |
| (2S)-2-amino-3-phenylpropanol |
| (2S)-2-Amino-3-phenyl-1-propanol |
| (-)-L-PHENYLALANINOL |
| (2S)-2-amino-3-phenylpropan-1-ol |
| L-phenylalaninol |
| (S)-β-Aminobenzenepropanol |
| phenylalaninol |
| Benzenepropanol, β-amino-, (βS)- |
| 2-amino-3-phenylpropan-1-ol |
| (S)-(−)-2-Amino-3-phenyl-1-propanol |
| N-(β-Hydroxyethyl)aniline |
| 2-Amino-3-Phenyl-Propan-1-Ol |
| 2-Anilinoethanol |
| H-Phe-ol |
| MFCD00004732 |
| (±)-phenylethanolamine |
| N-2-hydroxyethylaniline |
| L-Phenylglycinol |
 CAS#:63-91-2
CAS#:63-91-2 CAS#:7524-50-7
CAS#:7524-50-7 CAS#:2577-90-4
CAS#:2577-90-4 CAS#:6372-14-1
CAS#:6372-14-1 CAS#:3182-93-2
CAS#:3182-93-2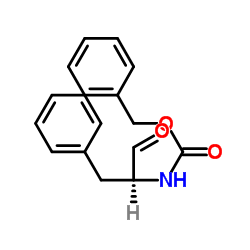 CAS#:59830-60-3
CAS#:59830-60-3 CAS#:150-30-1
CAS#:150-30-1 CAS#:2935-35-5
CAS#:2935-35-5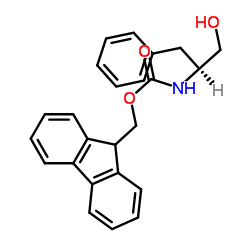 CAS#:129397-83-7
CAS#:129397-83-7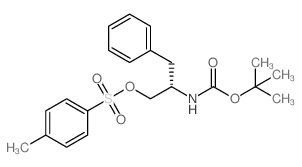 CAS#:141403-49-8
CAS#:141403-49-8![1-[(2S)-1-hydroxy-3-phenylpropan-2-yl]-3-phenylurea structure](https://image.chemsrc.com/caspic/384/223600-53-1.png) CAS#:223600-53-1
CAS#:223600-53-1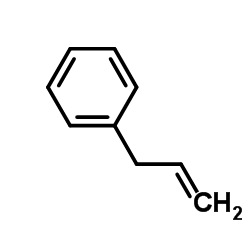 CAS#:300-57-2
CAS#:300-57-2 CAS#:93-54-9
CAS#:93-54-9 CAS#:1334326-63-4
CAS#:1334326-63-4 CAS#:29802-26-4
CAS#:29802-26-4 CAS#:82495-70-3
CAS#:82495-70-3 CAS#:90719-32-7
CAS#:90719-32-7 CAS#:2448-45-5
CAS#:2448-45-5![1-[4-[2-[(4-chlorophenyl)-phenylmethoxy]ethyl]piperazin-1-yl]propan-2-ol,oxalic acid structure](https://image.chemsrc.com/caspic/355/2323-36-6.png) CAS#:2323-36-6
CAS#:2323-36-6
