| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
L-Tryptophanol
CAS:2899-29-8 |
|
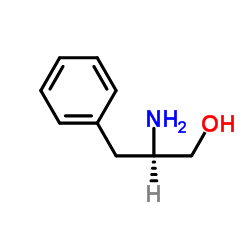 |
D-phenylalaninol
CAS:5267-64-1 |
|
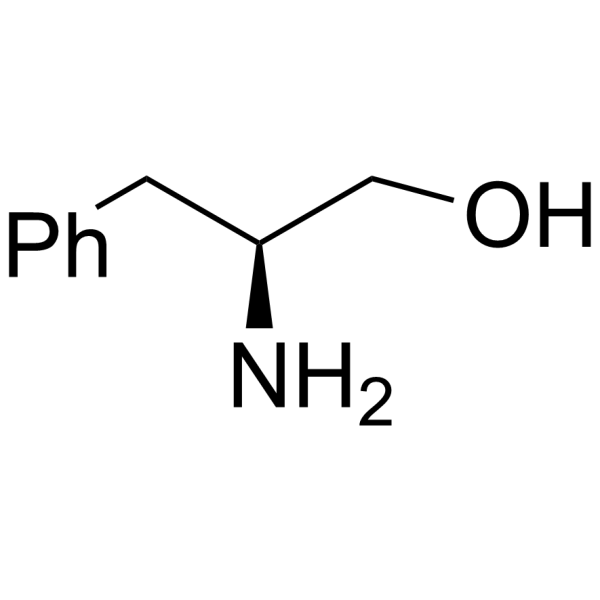 |
H-Phe-ol
CAS:3182-95-4 |