N-octyl alpha-D-glucopyranoside
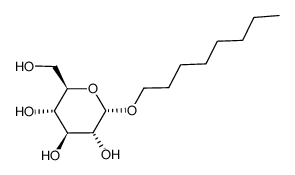
N-octyl alpha-D-glucopyranoside structure
|
Common Name | N-octyl alpha-D-glucopyranoside | ||
|---|---|---|---|---|
| CAS Number | 29781-80-4 | Molecular Weight | 292.36900 | |
| Density | 1.18 g/cm3 | Boiling Point | 454.1ºC at 760 mmHg | |
| Molecular Formula | C14H28O6 | Melting Point | 110-112ºC | |
| MSDS | Chinese USA | Flash Point | 228.4ºC | |
Use of N-octyl alpha-D-glucopyranosideOctyl α-D-glucopyranoside is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | octyl α-D-glucopyranoside |
|---|---|
| Synonym | More Synonyms |
| Description | Octyl α-D-glucopyranoside is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.18 g/cm3 |
|---|---|
| Boiling Point | 454.1ºC at 760 mmHg |
| Melting Point | 110-112ºC |
| Molecular Formula | C14H28O6 |
| Molecular Weight | 292.36900 |
| Flash Point | 228.4ºC |
| Exact Mass | 292.18900 |
| PSA | 99.38000 |
| LogP | 0.16340 |
| Vapour Pressure | 3.72E-10mmHg at 25°C |
| Index of Refraction | 1.515 |
| InChIKey | HEGSGKPQLMEBJL-RGDJUOJXSA-N |
| SMILES | CCCCCCCCOC1OC(CO)C(O)C(O)C1O |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 7 | |
|---|---|
| DownStream 7 | |
|
Charged surfactants induce a non-fibrillar aggregation pathway of amyloid-beta peptide.
J. Pept. Sci. 19(9) , 581-7, (2013) The amyloid β-peptide with a sequence of 42 amino acids is the major constituent of extracellular amyloid deposits in Alzheimer's disease plaques. The control of the peptide self-assembly is difficult... |
|
|
Carbohydrate recognition and photodegradation by an anthracene-Kemp's acid hybrid.
Org. Biomol. Chem. 10(42) , 8393-5, (2012) Selective recognition and photodegradation of a monosaccharide, octyl β-D-glucopyranoside, was achieved without any additives under neutral conditions using an anthracene-Kemp's acid hybrid and long-w... |
|
|
Improved technique for reconstituting incredibly high and soluble amounts of tetrameric K⁺ channel in natural membranes.
J. Membr. Biol. 241(3) , 141-4, (2011) The reconstitution of large amounts of integral proteins into lipid vesicles is largely prompted by the complexity of most biological membranes and protein stability. We optimized a particular system ... |
| octyl alpha-D-glucopyranoside |
| (2R,3S,4S,5R,6S)-2-(hydroxymethyl)-6-octoxyoxane-3,4,5-triol |
| MFCD00070008 |
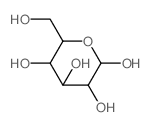 CAS#:2280-44-6
CAS#:2280-44-6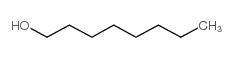 CAS#:111-87-5
CAS#:111-87-5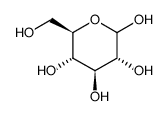 CAS#:492-62-6
CAS#:492-62-6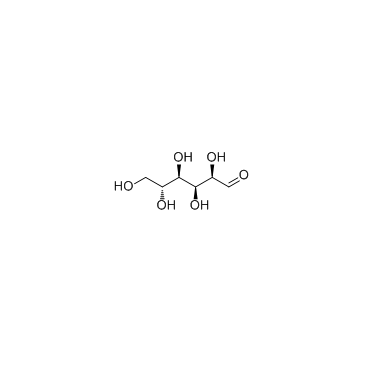 CAS#:50-99-7
CAS#:50-99-7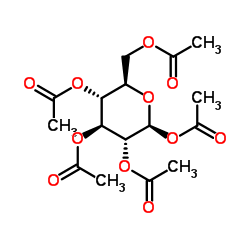 CAS#:604-69-3
CAS#:604-69-3 CAS#:9004-34-6
CAS#:9004-34-6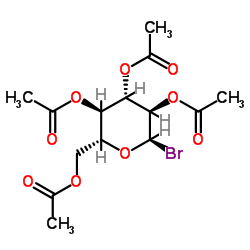 CAS#:572-09-8
CAS#:572-09-8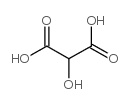 CAS#:80-69-3
CAS#:80-69-3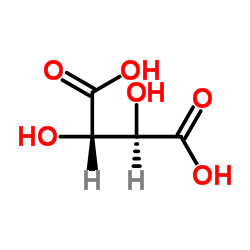 CAS#:147-73-9
CAS#:147-73-9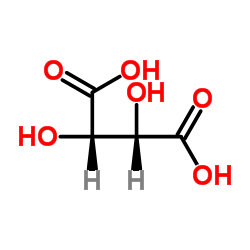 CAS#:87-69-4
CAS#:87-69-4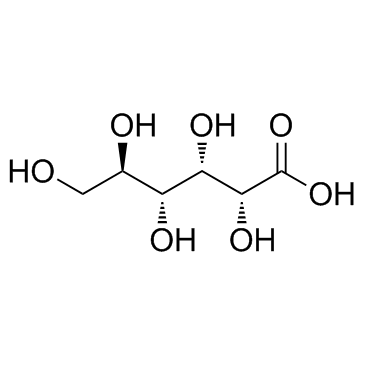 CAS#:526-95-4
CAS#:526-95-4 CAS#:87-73-0
CAS#:87-73-0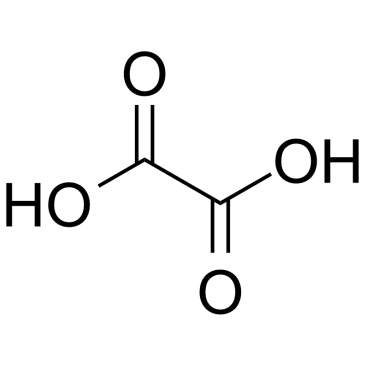 CAS#:144-62-7
CAS#:144-62-7 CAS#:124-07-2
CAS#:124-07-2
