L-685,458
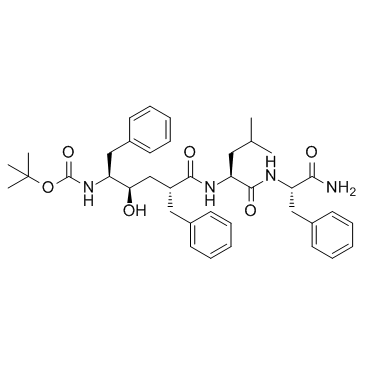
L-685,458 structure
|
Common Name | L-685,458 | ||
|---|---|---|---|---|
| CAS Number | 292632-98-5 | Molecular Weight | 672.85300 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C39H52N4O6 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of L-685,458L-685458 is a potent inhibitor of Amyloid β-Protein precursor γ-secretase activity with IC50 of 17 nM, shows greater than 50-100-fold selectivity over other aspartyl proteases tested.IC50 value: 17 nMTarget: γ-secretasein vitro: L-685458 is a Notch inhibitor. L-685458 blocks Notch activation in the two cell lines in terms of reduced cytoplasmic distribution and almost diminished nuclear labelling of Hes1 proteins. [2] L-685458 is a γ-secretase inhibitor. [3] |
| Name | L-685,458,(5S)-(tert-Butoxycarbonylamino)-6-phenyl-(4R)-hydroxy-(2R)-benzylhexanoyl)-L-leucy-L-phenylalaninamide |
|---|---|
| Synonym | More Synonyms |
| Description | L-685458 is a potent inhibitor of Amyloid β-Protein precursor γ-secretase activity with IC50 of 17 nM, shows greater than 50-100-fold selectivity over other aspartyl proteases tested.IC50 value: 17 nMTarget: γ-secretasein vitro: L-685458 is a Notch inhibitor. L-685458 blocks Notch activation in the two cell lines in terms of reduced cytoplasmic distribution and almost diminished nuclear labelling of Hes1 proteins. [2] L-685458 is a γ-secretase inhibitor. [3] |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C39H52N4O6 |
|---|---|
| Molecular Weight | 672.85300 |
| Exact Mass | 672.38900 |
| PSA | 159.85000 |
| LogP | 6.34900 |
| Storage condition | -20℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
RAGE inhibition in microglia prevents ischemia-dependent synaptic dysfunction in an amyloid-enriched environment.
J. Neurosci. 34(26) , 8749-60, (2014) Ischemia is known to increase the deleterious effect of β-amyloid (Aβ), contributing to early cognitive impairment in Alzheimer's disease. Here, we investigated whether transient ischemia may function... |
|
|
Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation.
Endocrinology 150 , 1014-24, (2009) Notch signaling directs cell fate during embryogenesis by influencing cell proliferation, differentiation, and apoptosis. Notch genes are expressed in the adult mouse ovary, and roles for Notch in reg... |
|
|
L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity.
Biochemistry 39 , 8698, (2000) Progressive cerebral amyloid beta-protein (A beta) deposition is believed to play a central role in the pathogenesis of Alzheimer's disease (AD). Elevated levels of A beta(42) peptide formation have b... |
| (5S)-(t-Butoxycarbonylamino)-6-phenyl-(4R)hydroxy-(2R)benzylhexanoyl)-L-leu-L-phe-amide |
| L-685458 |