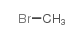atropine methyl bromide
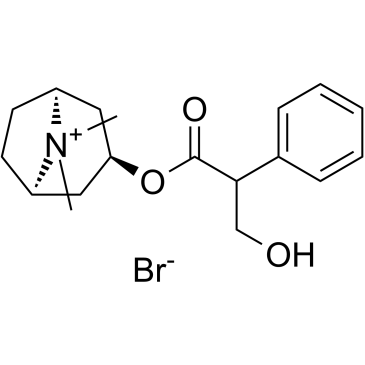
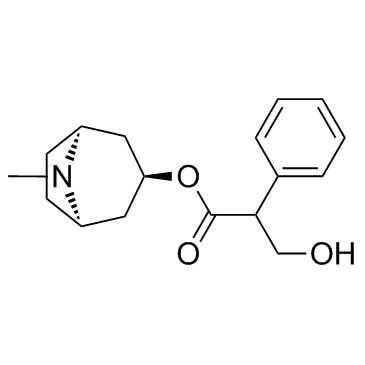
atropine methyl bromide structure
|
Common Name | atropine methyl bromide | ||
|---|---|---|---|---|
| CAS Number | 2870-71-5 | Molecular Weight | 384.31 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C18H26BrNO3 | Melting Point | 222-223° | |
| MSDS | N/A | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of atropine methyl bromideAtropine methyl bromide, a muscarinic receptor (mAChR) antagonist, is a quaternary ammonium salt of atropine and a mydriatic for dilation of the pupil during ophthalmic examination. It is introduced for relieving pyloric spasm in infants for its highly polar nature. It penetrates less readily into the central nervous system than atropine[1][2]. |
| Name | (8,8-dimethyl-8-azoniabicyclo[3.2.1]octan-3-yl) 3-hydroxy-2-phenylpropanoate,bromide |
|---|---|
| Synonym | More Synonyms |
| Description | Atropine methyl bromide, a muscarinic receptor (mAChR) antagonist, is a quaternary ammonium salt of atropine and a mydriatic for dilation of the pupil during ophthalmic examination. It is introduced for relieving pyloric spasm in infants for its highly polar nature. It penetrates less readily into the central nervous system than atropine[1][2]. |
|---|---|
| Related Catalog | |
| References |
[1]. Methylatropine. |
| Melting Point | 222-223° |
|---|---|
| Molecular Formula | C18H26BrNO3 |
| Molecular Weight | 384.31 |
| Exact Mass | 383.11000 |
| PSA | 46.53000 |
| Appearance of Characters | neat |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
atropine methyl... CAS#:2870-71-5 |
| Literature: Malpass American Journal of Pharmacy and the Sciences Supporting Public Health (1937-1978), 1951 , vol. 123, p. 5,8 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Bezold–Jarisch reflex in sino-aortic denervated malnourished rats
Auton. Neurosci. 162(1-2) , 48-53, (2011) In this study we assessed the role of Bezold–Jarisch reflex (BJR) in the regulation of blood pressure (BP) of malnourished (MN) and control rats (CN) with sino-aortic denervation (SAD). Fischer rats w... |
|
|
Frequency components of systolic blood pressure variability reflect vasomotor and cardiac sympathetic functions in conscious rats.
J. Physiol. Sci. 61(5) , 373-83, (2011) In this study, after confirming the suppression of autonomic nervous function by isoflurane anesthesia using autonomic antagonists, we pharmacologically investigated the involvement of vasomotor and c... |
|
|
Central and peripheral mechanisms underlying gastric distention inhibitory reflex responses in hypercapnic-acidotic rats.
Am. J. Physiol. Heart Circ. Physiol. 300(3) , H1003-12, (2011) We have observed that in chloralose-anesthetized animals, gastric distension (GD) typically increases blood pressure (BP) under normoxic normocapnic conditions. However, we recently noted repeatable d... |
| Hyoscyamine methylbromide |
| EINECS 220-700-1 |
| 8-Methylatropinium bromide |
| Methylatropine bromide |
| Mintussin |
| Methylatropinium bromide |
| Atropine methobromide |
| atropine methyl bromohydrate |
| 8,8-dimethyl-3endo-DL-tropoyloxy-nortropanium,bromide |
| MFCD00050293 |
| ATROPINE METHYL BROMIDE |
| Tropin |
| Mydriasin |
| N-Methylatropine bromide |
| 8,8-Dimethyl-3endo-DL-tropoyloxy-nortropanium,Bromid |