Boc-NH-C4-acid
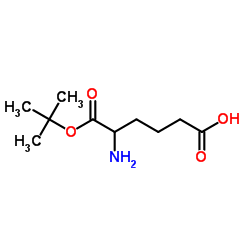
Boc-NH-C4-acid structure
|
Common Name | Boc-NH-C4-acid | ||
|---|---|---|---|---|
| CAS Number | 27219-07-4 | Molecular Weight | 217.262 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 331.7±27.0 °C at 760 mmHg | |
| Molecular Formula | C10H19NO4 | Melting Point | 48-52 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 154.4±23.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Boc-NH-C4-acidBoc-NH-C4-acid is a PROTAC linker, which belongs to a Alkyl/ether linker. Boc-NH-C4-acid can be used in the synthesis of the compound PROTAC1, and specifically degrades EED, EZH2, and SUZ12 in the PRC2 Complex. |
| Name | 5-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-NH-C4-acid is a PROTAC linker, which belongs to a Alkyl/ether linker. Boc-NH-C4-acid can be used in the synthesis of the compound PROTAC1, and specifically degrades EED, EZH2, and SUZ12 in the PRC2 Complex. |
|---|---|
| Related Catalog | |
| Target |
Alkyl/ether |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 331.7±27.0 °C at 760 mmHg |
| Melting Point | 48-52 °C(lit.) |
| Molecular Formula | C10H19NO4 |
| Molecular Weight | 217.262 |
| Flash Point | 154.4±23.7 °C |
| Exact Mass | 217.131409 |
| PSA | 75.63000 |
| LogP | 0.84 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.475 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2924199090 |
|
~95% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Gavande, Navnath; Kim, Hye-Lim; Doddareddy, Munikumar R.; Johnston, Graham A. R.; Chebib, Mary; Hanrahan, Jane R. ACS Medicinal Chemistry Letters, 2013 , vol. 4, # 4 p. 402 - 407 |
|
~47% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Lippard, Stephen J.; Barnes, Carmen M.; Haskel, Ariel; Barnes, Katie R. Patent: US2004/235712 A1, 2004 ; Location in patent: Page/Page column 34 ; |
|
~90% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Flynn, Daniel L.; Zelle, Robert E.; Grieco, Paul A. Journal of Organic Chemistry, 1983 , vol. 48, # 14 p. 2424 - 2426 |
|
~75% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Hartlieb, Matthias; Pretzel, David; Kempe, Kristian; Fritzsche, Carolin; Paulus, Renzo M.; Gottschaldt, Michael; Schubert, Ulrich S. Soft Matter, 2013 , vol. 9, # 18 p. 4693 - 4704 |
|
~94% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Ishida, Hideharu; Ogawa, Yuji; Imai, Yasuyuki; Kiso, Makoto; Hasegawa, Akira; et al. Carbohydrate Research, 1989 , vol. 194, p. 199 - 208 |
|
~% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Nisshin Flour Milling Co., Ltd. Patent: US6252041 B1, 2001 ; |
|
~% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Flynn, Daniel L.; Zelle, Robert E.; Grieco, Paul A. Journal of Organic Chemistry, 1983 , vol. 48, # 14 p. 2424 - 2426 |
|
~% 
Boc-NH-C4-acid CAS#:27219-07-4 |
| Literature: Flynn, Daniel L.; Zelle, Robert E.; Grieco, Paul A. Journal of Organic Chemistry, 1983 , vol. 48, # 14 p. 2424 - 2426 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| 5-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)pentanoic acid |
| 5-[(tert-Butoxycarbonyl)amino]pentanoic acid |
| 5-(N-t-butoxycarbonylamino)pentanoic acid |
| 5-[[(1,1-Dimethylethoxy)carbonyl]amino]-pentanoic acid |
| MFCD00076903 |
| 5-[(tert-Butoxycarbonyl)amino]valeric Acid |
| 5-(Boc-amino)valeric acid |
| Boc-5-Ava-OH |
| N-(tert-Butoxycarbonyl)-5-aminovaleric Acid |
| 2-Methyl-2-propanyl 6-hydroxy-6-oxonorleucinate |
| Hexanedioic acid, 2-amino-, 1-(1,1-dimethylethyl) ester |
| 5-(Boc-amino)pentanoic Acid |
| 5-(tert-Butoxycarbonylamino)pentanoic Acid |
| 5-(N-Boc-amino)valeric acid |
| N-Boc-5-aminovaleric Acid |
| 5-Boc-amino-pentanoic acid |
| 5-(tert-Butoxycarbonylamino)valeric Acid |
| N-Boc-5-aminopentanoic Acid |
| 5-(N-t-butoxycarbonylamino)valeric acid |
| Pentanoic acid, 5-[[(1,1-dimethylethoxy)carbonyl]amino]- |
| boc-5-aminovaleric acid |
| 5-(N-tert-butoxycarbonylamino)-pentanoic acid |
| 5-(N-t-butyloxycarbonylamino)-pentanoic acid |
| N-(tert-Butoxycarbonyl)-5-aminopentanoic Acid |
| Boc-5-aminopentanoic acid |


 CAS#:51644-96-3
CAS#:51644-96-3 CAS#:83948-54-3
CAS#:83948-54-3 CAS#:75178-90-4
CAS#:75178-90-4![8,9-DIHYDRO-6H-PYRIDO[2,1-B]QUINAZOLIN-11(7H)-ONE structure](https://image.chemsrc.com/caspic/232/2446-62-0.png) CAS#:2446-62-0
CAS#:2446-62-0 CAS#:88313-82-0
CAS#:88313-82-0 CAS#:91419-47-5
CAS#:91419-47-5![2-chloro-6,7,8,9-tetrahydropyrido[2,1-b]quinazolin-11-one structure](https://image.chemsrc.com/caspic/157/60811-47-4.png) CAS#:60811-47-4
CAS#:60811-47-4
